Extracellular matrix turnover is a major determinant of cardiac remodeling in heart failure. Sacubitril/valsartan decreases serum levels of procollagen type I amino-terminal peptide (PINP) and procollagen type III amino-terminal peptide (PIIINP), but its correlation with reverse remodeling is unknown. The purpose of this study is to determine the effect of sacubitril/valsartan on the profibrotic PINP and PIIINP biomarkers and their association with reverse cardiac remodeling in patients with heart failure with reduced ejection fraction (HFrEF).
MethodsProspective, single-center, open-label study of 401 outpatients with HFrEF initiated with sacubitril/valsartan treatment according to Guideline-Directed Medical Therapy. Change in serum levels of PINP, PIIINP, left ventricle end-diastolic volume index, left ventricle end-systolic volume index and left ventricle ejection fraction were analyzed from baseline to 12-month follow-up.
ResultsBaseline levels of PINP and PIIINP were highest (above-median) in 63 patients with ischemic cardiomyopathy and 19 with idiopathic dilated cardiomyopathy, decreasing −36μg/L for PINP (95%CI, 78–93; P<0.001), and −3.0μg/L for PIIINP (95%CI, 73–91; P<.001), at 12 months with sacubitril/valsartan therapy. These changes were correlated with an improvement in left ventricle ejection fraction from 29% to 36%, and a reduction in left ventricle end-diastolic (−20mL/m2) and end-systolic (−18mL/m2) volumes in this subgroup (P<.001). Advanced age, diabetes, hypertension, and greater dilation of left ventricle were more frequent in this subgroup (P<.001).
ConclusionsThese findings suggest that changes in fibrosis markers induced by sacubitril-valsartan therapy in HFrEF are related to reverse cardiac remodeling in patients with higher PINP and PIIINP levels.
El recambio de la matriz extracelular es determinante en el proceso de remodelado cardiaco. Sacubitrilo-valsartán disminuye el péptido aminoterminal del procolágeno tipo I (PINP) y tipo III (PIIINP), pero se desconoce su correlación con el remodelado reverso. El objetivo es determinar el efecto del sacubitrilo-valsartán sobre los biomarcadores PINP y PIIINP, y su asociación con el remodelado reverso en pacientes con insuficiencia cardiaca con fracción de eyección reducida (ICFEr).
MétodosEstudio prospectivo de 401 pacientes con ICFEr tratados con sacubitrilo-valsartán, de acuerdo a las guías de práctica clínica. Se analizaron los cambios en PINP, PIIINP, fracción de eyección y volúmenes indexados de fin de diástole y fin de sístole del ventrículo izquierdo al inicio y a los 12 meses de seguimiento.
ResultadosLos niveles basales de PINP y PIIINP fueron más altos (por encima de la media) en 63 pacientes con miocardiopatía isquémica y 19 con miocardiopatía dilatada idiopática, disminuyendo −36μg/l para PINP (IC95%, 78-93; p<0,001) y −3,0μg/l para PIIINP (IC95%, 73-91; p<0,001), a los 12 meses de tratamiento con sacubitrilo-valsartán. Estos cambios se correlacionaron con aumento en la fracción de eyección del ventrículo izquierdo del 29 al 36% y reducción en los volúmenes de fin de diástole (−20ml/m2) y fin de sístole (−18ml/m2). La edad avanzada, la diabetes, la hipertensión y la mayor dilatación del ventrículo izquierdo fueron más frecuentes en este subgrupo (p<0,001).
ConclusionesLos cambios en los biomarcadores inducidos por sacubitrilo-valsartán en ICFEr se correlacionan con el remodelado reverso en pacientes con niveles más altos de PINP y PIIINP.
Cardiac remodeling is an essential process in the progression of heart failure (HF). Remodeling is defined as any changes to cardiac chamber geometry and function and may occur in response to sustained physiological or pathophysiological stimuli such as cardiac injury, hemodynamic changes, inflammation and neurohormonal activation. Although it is initially adaptive in the short-term, in the long-term it increases cardiac work, promotes hypertrophy, activates fibroblasts, and contributes to the development of myocardial fibrosis. The concept of cardiac remodeling was initially developed to describe changes, which occur in the days and months following myocardial infarction, but it has been extended to cardiomyopathies of non-ischemic origin, such as idiopathic dilated cardiomyopathy or hypertensive cardiomyopathy, suggesting common mechanism for the progression of cardiac dysfunction.1–5 During remodeling in HF with reduced ejection fraction (HFrEF), the left ventricle manifests as gradual increases in end-diastolic and end-systolic volumes, wall thinning, and a change in chamber geometry from an ellipsoidal shape to a more spherical, less elongated shape. These processes are associated with worsening systolic or diastolic functions and are accompanied by changes in extracellular matrix (ECM). Such changes are termed remodeling, whereas reverse remodeling occurs when left ventricle geometry and/or function reverts closer to that of normal heart structure and is associated with improved cardiac function and better prognosis.6–10
Reverse remodeling ussualy occurs in the context of intensification and titration of drug and device therapy, but the magnitude of left ventricle recovery is far from consistent across patients. This is partly determined by specific factors related to the cause and duration of HF. Advanced age, ischemic causes, presence of left bundle branch block, diagnosis of HF dating from > 12 months, higher concentrations of cardiac biomarkers, and lack of guideline-directed HF therapy are associated with adverse remodeling. Interestingly, although lower left ventricle ejection fraction (LVEF), larger left ventricle volumes, and higher levels of fibrosis markers are associated with worse prognosis (high hospital admissions, mortality, or the need for a heart transplant), studies suggest that these patients may have greater propensity for reverse remodeling.11–14 Identification of reliable predictors of reverse remodeling would enable cardiologists to adopt a more tailored approach in this high risk population.
The PARADIGM-HF (Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor With Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure trial) provided a unique opportunity to examine a panel of biomarkers that reflect ECM homeostasis in a large cohort of 1776 well-characterized HFrEF patients that included long-term outcome data. In the analysis, at baseline the profibrotic biomarkers procollagen type I amino-terminal peptide (PINP) and procollagen type III amino-terminal peptide (PIIINP), were higher than published referent control values. The higher the baseline of PINP (> 40μg/L), and PIIINP (> 6.0μg/L), the higher the subsequent rates of cardiovascular death and HF hospitalizations. Eight months after randomization sacubitril/valsartan significantly decreased these biomarkers compared with enalapril treatment (P<.05). Although reduction in profibrotic biomarkers was strongly associated with outcomes, a link to reverse cardiac remodeling was not examined. In aggregate, these data suggest that one mechanism by wich sacubitril/valsartan may exert a beneficial outcome in HFrEF patientes may be related to a reduction in profibrotic signaling.15
Recently, the PROVE-HF (Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure), showed a weak reduction in N-terminal pro-B-type natriuretic peptide in 654 patients with HFrEF treated with sacubitril/valsartan (from 816pg/mL at baseline to 455pg/mL at 12 months), and was associated with significant improvement in left ventricle volumes and LVEF by mechanisms that are not fully understood.16 These findings suggest that the effects of sacubitril/valsartan on reverse cardiac remodeling in HFrEF patients are not exclusively related to angiotensin receptor antagonism and neprilysin inhibition.
Since cardiac remodeling is an important aspect of disease progression, preventing or reversing maladaptive remodeling is an accepted therapeutical target. The purpose of this study is to determine the effect of sacubitril/valsartan on the profibrotic PINP and PIIINP biomarkers and their association with reverse cardiac remodeling in patients with HFrEF.
MethodsThis is an observational, prospective, open-label, single-center study, of 401 outpatients with HFrEF of ischemic or idiopathic etiology and history of New York Heart Association class II, III or IV initiated with sacubitril/valsartan treatment according to Guideline-Directed Medical Therapy of Heart Failure. The diagnosis of ischemic cardiomyopathy and idiopathic dilated cardiomyopathy was made according to a standardized definition for use in clinical research,17 and World Health Organization criteria.18 Reverse cardiac remodeling was defined as an absolute LVEF increase>10% and relative percent left ventricle end-diastolic volume reduction>10%.19 To be eligible, they had to have been receiving for at least 2 weeks a fixed-dose drug regimen that could include angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers, mineralocorticoid receptor antagonists and diuretics, and were clinically stable. Enrollment commenced on January 31, 2017, and follow-up was completed on January 15, 2020. Patients with recent tricameral pacemaker implantation (< 6 months), hospitalization for HF (< 1 month), or with other concomitant diseases such as liver cirrhosis, hyperthyroidism, pulmonary fibrosis, chronic kidney failure, cancer or autoimmune diseases, were excluded. This study was approved by the DIME Clínica Neurocardiovascular ethics committee and an informed, written consent was obtained of each patient. Following informed consent, angiotensin/converting enzyme inhibitors and angiotensin receptor blockers were discontinued, and patients were given sacubitril/valsartan according to the European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure.20 Following angiotensin receptor antagonism and neprilysin inhibition initiation, patients returned for drug titration every 2 weeks, with a goal dose of sacubitril/valsartan of 97/103mg twice daily or highest tolerated dose. The criteria for increasing the dose included a systolic blood pressure of 90mmHg or higher while the patient was standing, the absence of symptoms of hypotension, and a serum creatinine concentration of less than 2.0mg per deciliter or no more than 50 percent higher than the base-line concentration. Patients returned for follow-up visits at 2, 4, and 6 months and every 3 months thereafter. Treatment continued for at least to 12 months. Blood samples were obtained and 2-dimensional echocardiography was performed at baseline and at 12-month follow-up. Clinical data, cardiovascular risk factors (hypertension, diabetes mellitus), medication (dose, frequency), and ventricular parameters, were entered into a database.
Patients were included in the study if they fulfill the following criteria: a) Age>18 years. b) HF of ischemic or idiopathic etiology (New York Heart Association class II–IV). c) LVEF<40%. d) Last hospitalization due to HF (> 1 month). e) Three-chamber pacemaker implantation (> 6 months).
Patients were excluded from the study if they present any of the following conditions: a) Right HF due to chronic cor-pulmonale. b) HF of other than ischemic or idiopathic etiology. c) Pregnancy. d) Chronic kidney failure (creatinine>3mg/dL). e) Liver cirrhosis. f) Hyperthyroidism. g) Amyloidosis. h) Pulmonary fibrosis. i) Autoimmune diseases.
Blood sample collectionBlood samples were collected from the antecubital region (venipuncture) on the day the patient was enrolled and at 12 months. Serum was stored at −70°C until its analysis. Except for the first one, blood extractions were performed so as to coincide with scheduled controls, and therefore did not entailed any additional intervention. All baseline and 12-month follow-up samples were analyzed at once. Using immunoassays from commercially available kits, we measured 2 ECM serum markers: PINP) and PIIINP.
Determination of procollagen type I amino-terminal peptide (PINP)A 350μL volume of each sample was put into sterile plastic microcentrifuge tubes. The serum was centrifuged at 1.300rpm for 10min at 4°C. The levels of PINP were determined using the Elecsys 2010 automatic analyzer (Roche Diagnostics, Switzerland). The “El ecsys total PINP” test was used by electrochemiluminescence immunoassay (ECLIA). The principle of the test is based on the use of a specific anti-PINP monoclonal biotinylated antibody and a ruthenium chelate-labeled anti-PINP monoclonal specific antibody, to which streptavidin-coated microparticles are incorporated, forming a complex between biotin and streptavidin. The mixture is transferred to a reading cell where, by magnetism, the microparticles are temporarily fixed to the surface of the electrode. The unfixed elements are subsequently removed by washing with the “ProCell” reagent. Applying a defined electric current produces a chemiluminescent reaction whose light emission is measured directly with a photomultiplier. The results are obtained by means of a calibration curve made in the system by reference to 2 points and a master curve included in the barcode of the reagent. The incubation time used to obtain a result was 18minutes, with a measurement interval of 5–1.200μg/L. Concentrations above the detection limit can be diluted with a recommended dilution of 1:2. The intra-assay precision was 2.3% and the inter-assay 2.8%.21
Determination of procollagen type III amino-terminal peptide (PIIINP)A volume of 300μL of each sample was placed in sterile plastic microcentrifuge tubes. The serum was centrifuged at 1.300rpm for 10min at 4°C, the supernatant was isolated. Before the test, both the samples and the reagents were 30min at room temperature. The levels of PIIINP were determined by means of a competitive radioimmunoassay using the UniQ PIIINP RIA kit, (Orion Diagnostics). The principle of the test is that a known amount of radiolabelled PIIINP (I125) and an unknown amount of PIIINP (without radioactive tracer) in the sample compete for the high-affinity binding sites of the antibody. After separating the free antigen, the amount of labeled PIIINP in the sample is inversely proportional to the amount of PIIINP contained in the serum. The results were obtained in terms of concentration (μg/L) from a log-logit representation of the calibration curve. The standard curve includes 7 polypropylene tubes with reconstituted human serum as calibrators: tube A (maximum junction) 0μg/L of PIIINP, tube B with 1.0μg/L, tube C with 2.5μg/L, tube D with 5.0μg/L, tube E with 10μg/L, tube F with 25μg/L and tube G with 50μg/L. The measurement range of the assay is 1.0–50μg/L. The mean intra-assay precision was 4.7% and the inter-assay 6.1%.21
EchocardiographyA complete resting 2-dimensional (2D) echocardiogram and Doppler ultrasound examination, including all standard views, was performed with a 3.5-MHz transducer (Philips IE33 Ultrasound System, United States) at baseline and at 12 months. Ejection fraction was derived from left ventricle volumes using the area-length method (modified Simpson's rule) in the apical 4- and 2-chamber views, as recommended by the American Society of Echocardiography and the European Association of Cardiovascular Imaging.22 Left ventricular end-diastolic and end-systolic volumes were indexed according to body surface. Two experienced readers, blinded to the clinical history, physical examination, laboratory data, and outcome variables, interpreted all echocardiograms and verified left ventricular volumetric analyses.
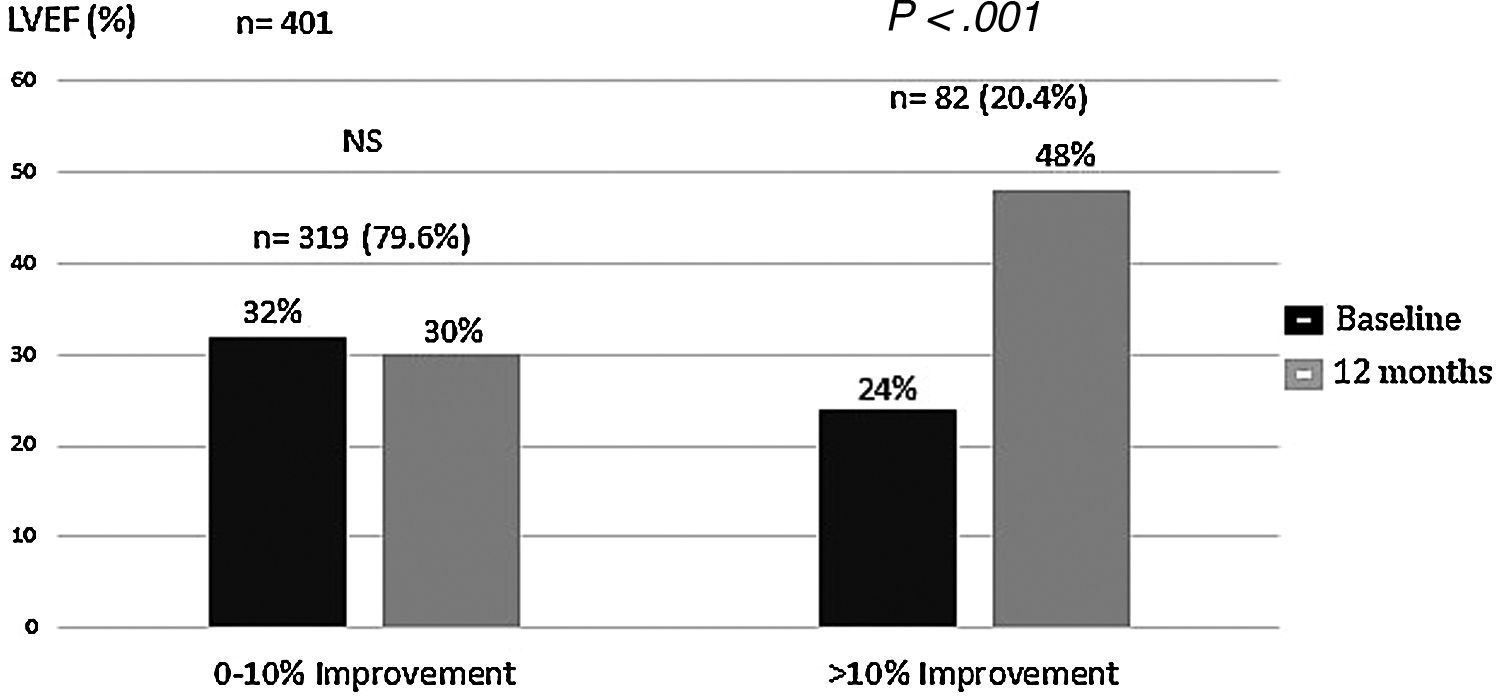
Statistical analysisData are presented as mean±standard deviation. A preliminary analysis was performed to detect outliers and decide on the most specific measure to describe the data. Because PINP and PIIINP values were not normally distributed, the data were logarithmic (ln) transformed prior to statistical analysis. Patients were grouped according to percentage of LVEF improvement (0%–10% and > 10%). Baseline measurements of left ventricular end-diastolic volume index and left ventricular end-systolic volume index were grouped according to quartiles of ventricular dilation. The treatment effects with sacubitril/valsartan were analyzed within the baseline percentages and quartiles. Change from baseline values of PINP and PIIINP at 12 months were based on least-squares mean values by analysis of covariance (ANCOVA). In all cases, a P value<.05 was considered with statistical significance. Data were analyzed with SPSS software, version 14.0 (Statistical Package for Social Sciences, SPSS Inc., United States).
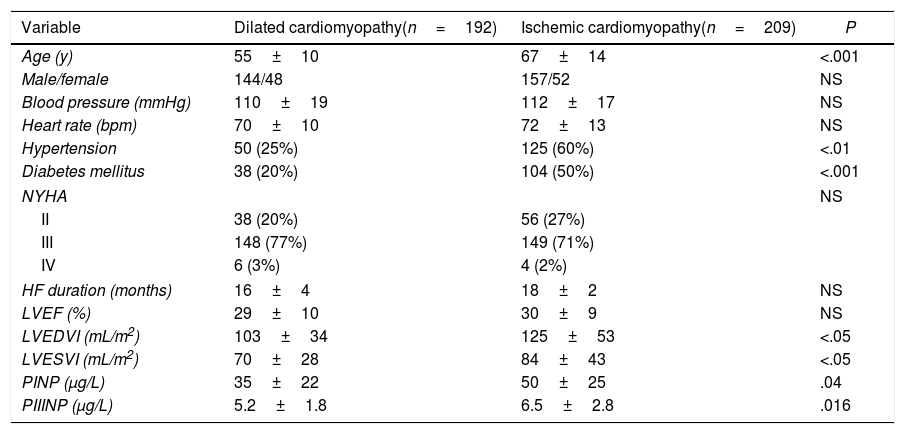
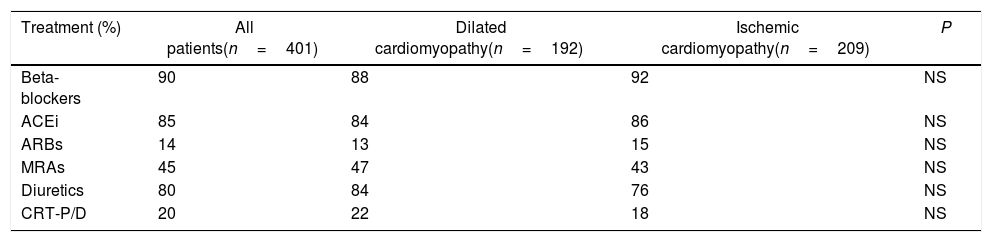
ResultsBaseline characteristics of the population are detailed in Table 1. Of the 401 patients included in the present study, 192 had idiopathic dilated cardiomyopathy, and 209 had ischemic cardiomyopathy. The mean age (standard deviation) was 61±12 years, being significantly higher in the ischemic cardiomyopathy group (67±14 vs 55±10; P<.001). Gender, LVEF and HF duration were similar in both groups. At enrollment, nearly all had New York Heart Association Class II (n=94; 23%) or III (n=297; 74%) symptoms. Diabetes and hypertension were more frequent in the ischemic group (P<.001). In the whole population, the median baseline levels were 40μg/L for PINP and 6.2μg/L for PIIINP, being significantly higher in ischemic cardiomyopathy compared with dilated cardiomyopathy (PINP 50±25 vs 35±22, P=.04; PIIINP 6.5±2.8 vs 5.2±1.8, P<.016). The ischemic cardiomyopathy group had worse left ventricular volumes (P<.05). There were no significant differences in previous pharmacological treatment and devices use (Table 2). The target dose of sacubitril/valsartan was achieved in 84 percent of the patients.
Baseline characteristics.
| Variable | Dilated cardiomyopathy(n=192) | Ischemic cardiomyopathy(n=209) | P |
|---|---|---|---|
| Age (y) | 55±10 | 67±14 | <.001 |
| Male/female | 144/48 | 157/52 | NS |
| Blood pressure (mmHg) | 110±19 | 112±17 | NS |
| Heart rate (bpm) | 70±10 | 72±13 | NS |
| Hypertension | 50 (25%) | 125 (60%) | <.01 |
| Diabetes mellitus | 38 (20%) | 104 (50%) | <.001 |
| NYHA | NS | ||
| II | 38 (20%) | 56 (27%) | |
| III | 148 (77%) | 149 (71%) | |
| IV | 6 (3%) | 4 (2%) | |
| HF duration (months) | 16±4 | 18±2 | NS |
| LVEF (%) | 29±10 | 30±9 | NS |
| LVEDVI (mL/m2) | 103±34 | 125±53 | <.05 |
| LVESVI (mL/m2) | 70±28 | 84±43 | <.05 |
| PINP (μg/L) | 35±22 | 50±25 | .04 |
| PIIINP (μg/L) | 5.2±1.8 | 6.5±2.8 | .016 |
HR, heart failure; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricle ejection fraction; LVESVI, left ventricular end-systolic volume index; NYHA, New York Heart Association functional class; PINP, procollagen type I N-terminal propeptide; PIIINP, procollagen type III N-terminal propeptide. Data are expressed as mean±standard deviation or percentage.
Pharmacological treatment and use of devices at enrollment.
| Treatment (%) | All patients(n=401) | Dilated cardiomyopathy(n=192) | Ischemic cardiomyopathy(n=209) | P |
|---|---|---|---|---|
| Beta-blockers | 90 | 88 | 92 | NS |
| ACEi | 85 | 84 | 86 | NS |
| ARBs | 14 | 13 | 15 | NS |
| MRAs | 45 | 47 | 43 | NS |
| Diuretics | 80 | 84 | 76 | NS |
| CRT-P/D | 20 | 22 | 18 | NS |
ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; MRA, mineralocorticoid receptor antagonists; CRT-P/D, cardiac resynchronization therapy pacemaker or defibrillator.
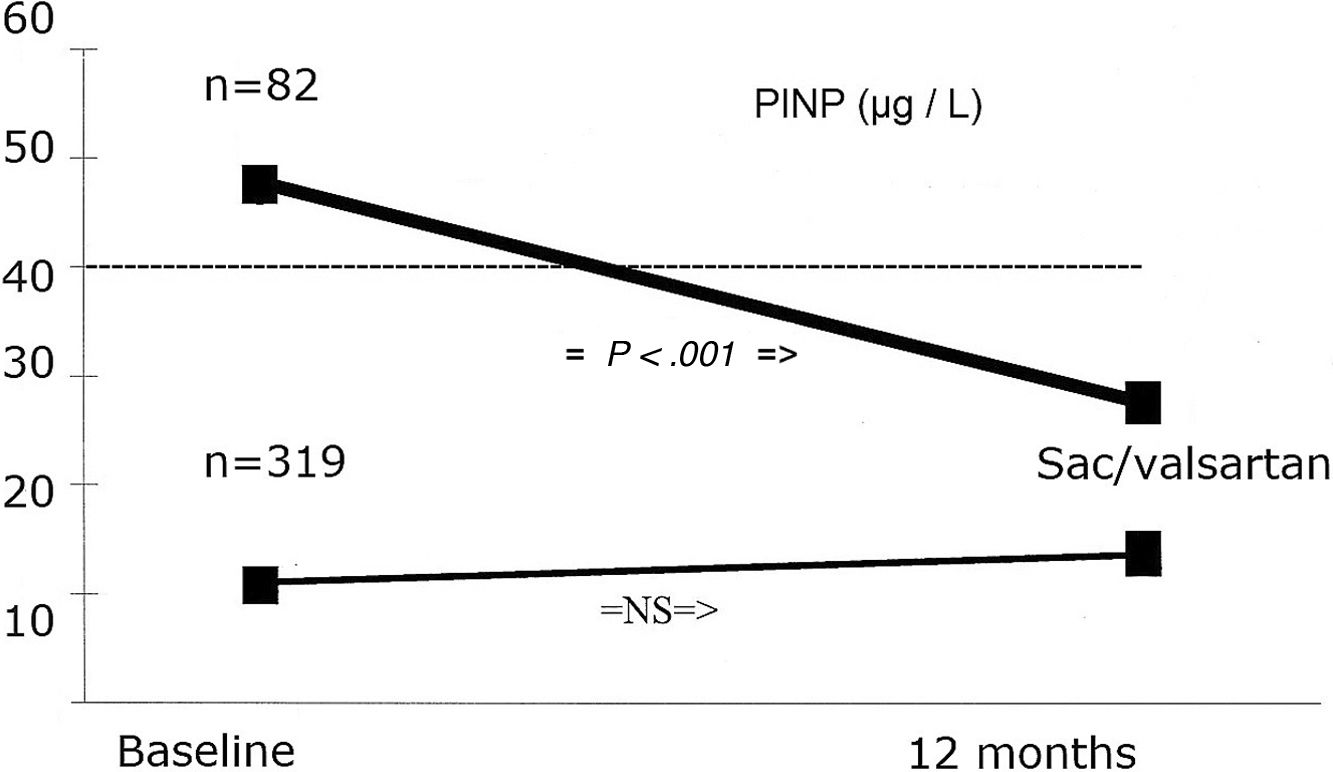
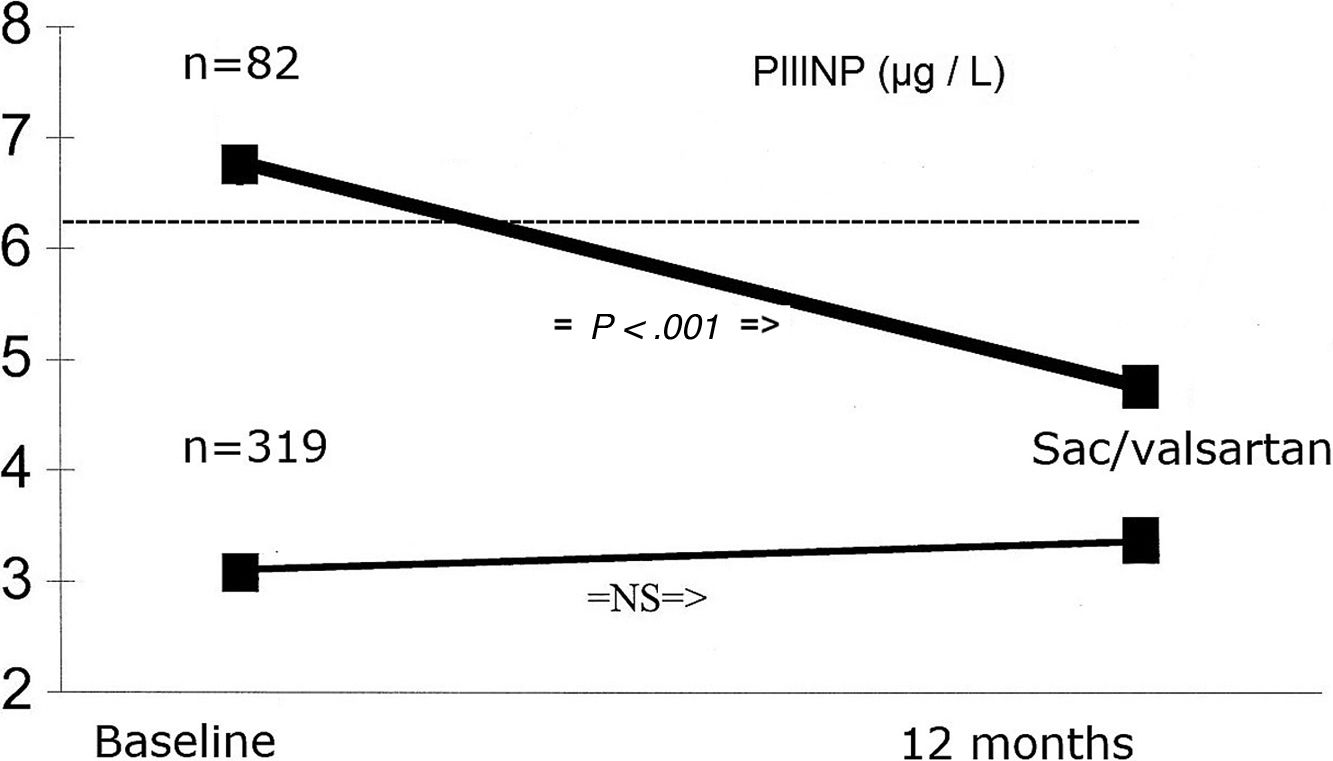
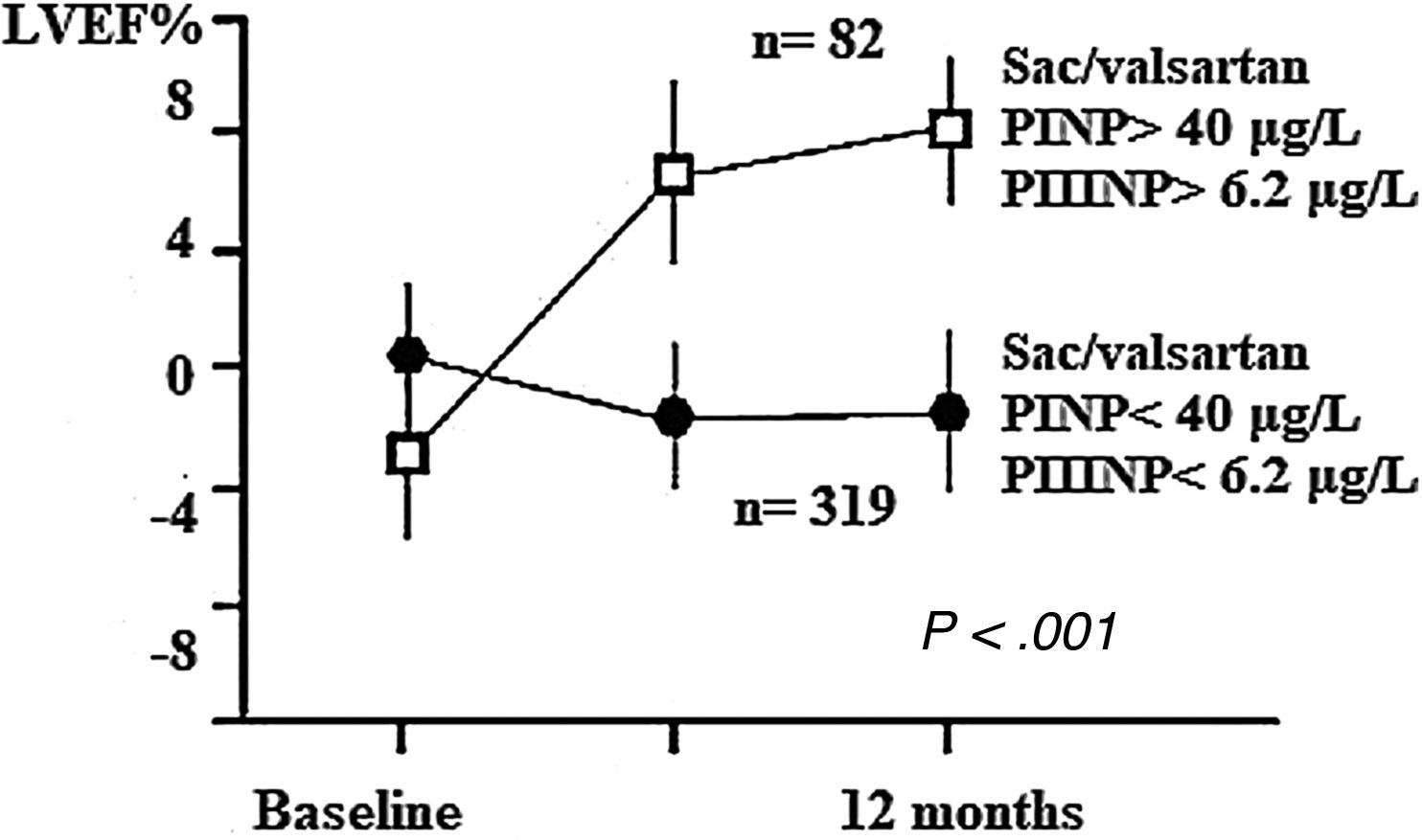
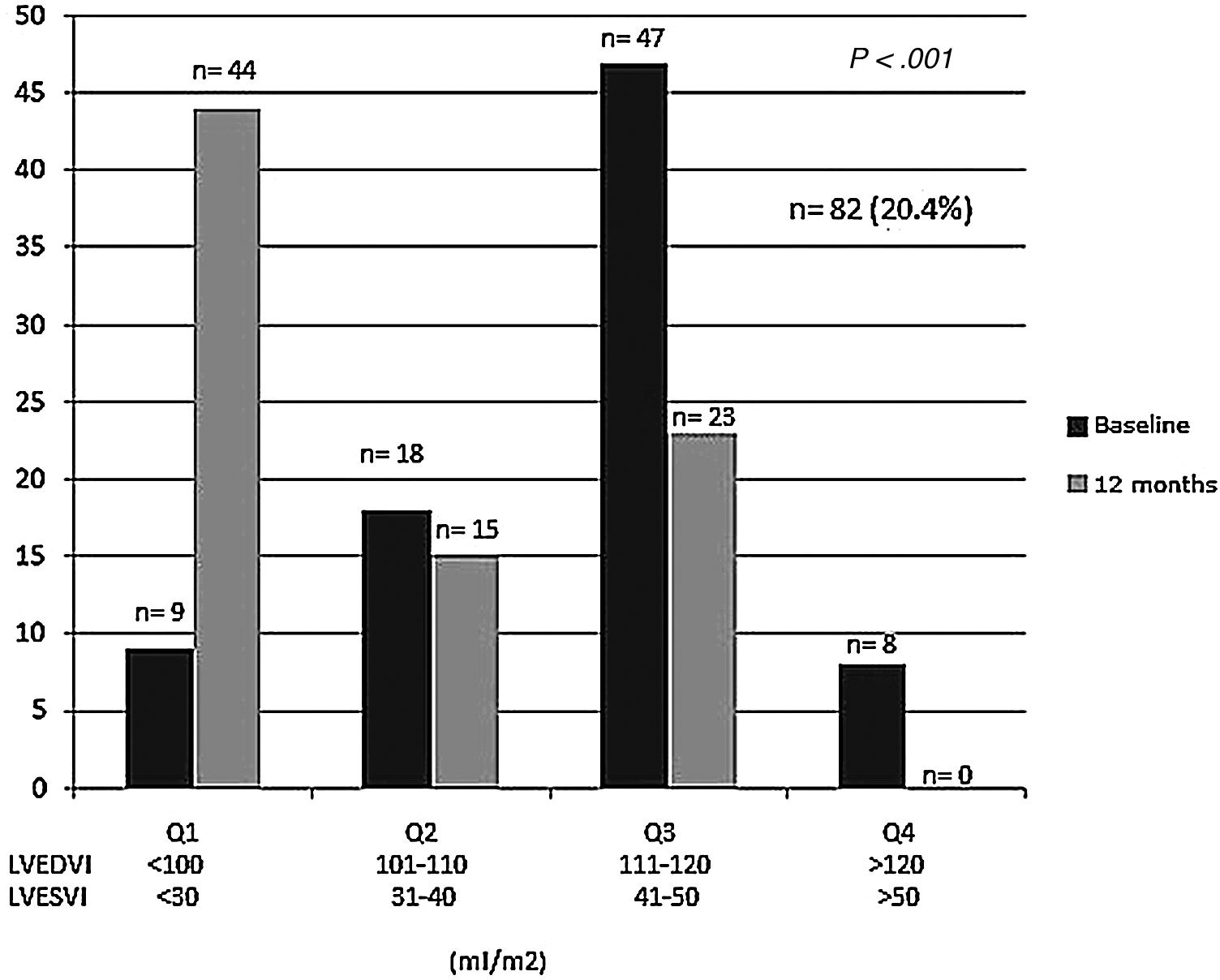
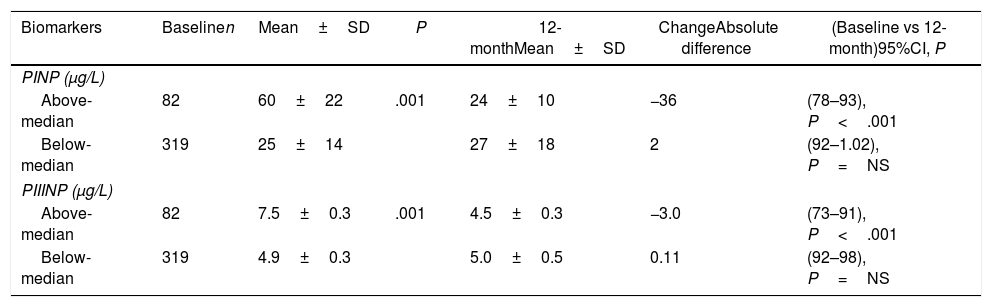
We analyzed changes in markers in subgroups with low (below-median) and high (above-median) levels in the whole population. When PINP and PIIINP baseline levels were below median, they did not change over 12 months with sacubitril/valsartan therapy (n=319), but when PINP and PIIINP baseline levels were above median, they decreased significantly at 12 months in 82 patients, 63 with ischemic cardiomyopathy and 19 with idiopathic dilated cardiomyopathy, of which 52(63%) with age 70 years or older, observing an absolute difference of −36μg/L for PINP (from 60±22 to 24±10; 95%CI, 78–93; P<.001), and -3.0μg/L for PIIINP (from 7.5±0.3 to 4.5±0.3; 95%CI, 73–91; P<.001) (Table 3, Figs. 1 and 2). A significant improvement in LVEF was observed in this subgroup with an absolute increase>10%. Baseline LVEF doubled on average, from 24% to 48% (P<.001), and the mean LVEF for that cohort increased from 29% at baseline to 36% (+7%) at 12 months (P<.001) (Figs. 3 and 4). The increase in LVEF in this subgroup of patients was correlated with decrease in left ventricle volumes. The absolute magnitude of change in left ventricle end-diastolic and end-systolic volumes was greatest in the quartile 4 (left ventricular end-diastolic volume index>120mL/m2 and left ventricular end-systolic volume index>50mL/m2), with a significant reduction in left ventricular end-diastolic volume index (−20mL/m2) and left ventricular end-systolic volume index (−18mL/m2) over 12 months (P<.001). Nonetheless, in each quartile, the magnitude of left ventricle volumes decreased (Fig. 5). These findings suggest that changes in fibrosis markers induced by sacubitril/valsartan therapy are related to reverse cardiac remodeling in those patients with higher PINP and PIIINP levels, advanced age, ischemic etiology, comorbid medical conditions (diabetes, hypertension) and severe dilation of left ventricle.
Serum levels of cardiac extracellular matrix markers at baseline and at 12-month follow-up.
| Biomarkers | Baselinen | Mean±SD | P | 12-monthMean±SD | ChangeAbsolute difference | (Baseline vs 12-month)95%CI, P |
|---|---|---|---|---|---|---|
| PINP (μg/L) | ||||||
| Above-median | 82 | 60±22 | .001 | 24±10 | −36 | (78–93), P<.001 |
| Below-median | 319 | 25±14 | 27±18 | 2 | (92–1.02), P=NS | |
| PIIINP (μg/L) | ||||||
| Above-median | 82 | 7.5±0.3 | .001 | 4.5±0.3 | −3.0 | (73–91), P<.001 |
| Below-median | 319 | 4.9±0.3 | 5.0±0.5 | 0.11 | (92–98), P=NS | |
PINP: procollagen type I N-terminal propeptide; PIIINP: procollagen type III N-terminal propeptide.
Data are expressed as mean±standard deviation (SD) according to above-median or below-median baseline levels (in micrograms per liter).
P-value: ANCOVA adjusted by baseline values.
Changes in left ventricular ejection fraction (LVEF) from baseline during the 12-month period of observation. The sacubitril/valsartan effect on LVEF was significant only in patients with above-median baseline levels (PINP>40μg/L, PIIINP>6.2μg/L). The overall mean LVEF increased from 29% at baseline to 36% (+7%) at 12 months (P<.001). PINP, procollagen type I N-terminal propeptide; PIIINP, procollagen type III N-terminal propeptide; Sac, sacubitril.
Changes in left ventricular end-diastolic and end-systolic volumes index from baseline during the 12-month period of observation according to quartiles Q1–Q4, defined by increasing severity of ventricular dilation from mild to severe. Reversal of left ventricular dilation is observed with sacubitril/valsartan therapy (P<.001). LVEDVI, left ventricular end-diastolic volume index; LVESVI, Left ventricular end-systolic volume index.
Cardiac remodeling is initially an adaptive response in heart failure, but it gradually becomes maladaptive, leading to fibrosis and disease progression.25 Multiple biomarkers of cardiac fibrosis have been investigated in the last 2 decades, of which very few have been of clinical utility. In this sense, for a circulating molecule to be considered a biochemical marker of myocardial remodeling, it must meet several criteria. First, there must be a relationship between its expression in the myocardium and its concentration in blood; second, there must be an association of its concentration in blood with the structural and/or functional cardiac parameters that reflect the characteristics of the myocardial change that is being studied and, finally, its levels must vary in parallel with the changes in the parameters previously mentioned, induced by pharmacological treatment.23 Measurement of ECM turnover using markers such as PINP and PIIINP is a useful tool for monitoring cardiac tissue repair. In the Randomized Aldactone Evaluation (RALES) study, 1663 patients with severe symptoms of HF and LVEF<35% were assigned to receive spironolactone or placebo. There was a 30% reduction in mortality in the spironolactone group (HR, 0.70; 95%CI, 0.60–0.82; P<.001). A subanalysis evaluated samples from 261 patients to determine their fibrotic status through the measurement at baseline serum levels of PINP and PIIINP and at 6-month follow-up. At 6 months these markers decreased in the spironolactone group but remained unchanged in the placebo group. The benefits in terms of morbidity and mortality associated with the use of spironolactone were greater in those patients with higher levels of these markers.24,25 In the analysis of the PARADIGM-HF study, at baseline PINP and PIIINP were higher than published referent control values. A subanalysis evaluated samples from 1776 patients with HFrEF measuring the basal serum levels of PINP and PIIINP and at 8-month follow-up. At 8 months, these markers decreased significantly in the sacubitril/valsartan group compared to the enalapril group, and exerted a beneficial outcome related to changes in these biomarkers.15 These findings are interesting because it is derived from them that there is a subgroup of potential “super-responders”, represented by patients with higher markers of collagen synthesis. In this context, we studied the relationship between changes in precursors of collagen synthesis (PINP and PIIINP) and changes in parameters for reverse remodeling (LVEF, left ventricular end-diastolic volume index and left ventricular end-systolic volume index) from baseline to 12-month follow-up, in patients con HFrEF of ischemic and dilated etiology treated with sacubitril/valsartan.
In our results, REMODELING HF (Reduction of Extracellular Matrix Markers and Reverse Cardiac Remodeling During Sacubitril/Valsartan Therapy for Heart Failure), only patients with higher serum levels of PINP and PIIINP had a significant decrease in these collagen synthesis markers with sacubitril/valsartan, and this decrease was correlated with reverse cardiac remodeling that showed an absolute increase in LVEF>10% and relative percent left ventricle end-diastolic volume reduction>10%, especially in older patients, ischemic cardiomyopathy, comorbid medical conditions (diabetes, hypertension), and greater dilation of the left ventricle. These findings demonstrate that the sacubitril/valsartan effects on outcomes in patients with HF are not exclusively related to angiotensin receptor antagonism and neprilysin inhibition, but rather that it has a direct effect on the ECM turnover, decreasing significantly the precursors of collagen synthesis in patients with HFrEF and an active fibrotic process.
These results could be important for clinicians and patients, because quantifying the levels of fibrosis markers in a non-invasive way accompanied by a biomarker-guided treatment may be an opportunity to decrease cardiac remodeling in patients with HFrEF with the use of antagonism and neprilysin inhibition, thus becoming a novel, non-expensive and easy to use and to implant method at medical centers and hospitals.
LimitationsThe main limitation of the present study is related to the absence of a control group; since sacubitril/valsartan has a class I clinical practice recommendation for HFrEF, which made a comparison group unethical. However, we believe that changes in fibrosis markers and its association with reverse cardiac remodeling are consistent with the time of treatment with sacubitril/valsartan.
ConclusionsFor the first time, we observe that changes in fibrosis markers induced by sacubitril/valsartan therapy in HFrEF are related to reverse cardiac remodeling in patients with higher PINP and PIIINP levels, advanced age, ischemic etiology, comorbid medical conditions, and severe left ventricle dilation. These findings suggest that there may be an opportunity to decrease adverse remodeling with the use of angiotensin receptor-neprilysin inhibitor, as a biomarker-guided therapeutic approach aimed at decreasing fibrogenic activity and modulate the remodeling process.
Extracellular matrix turnover has been recognized an important mediator of cardiac fibrosis and ventricular remodeling in heart failure, usually associated with an increase in the synthesis of types I and III collagen fibers. However, there are no studies that have specifically evaluated the impact of reduced collagen synthesis on reverse cardiac remodeling. Only with a better understanding of this process we will be able to develop new therapeutic approaches.
Does it contribute anything new?Sacubitril/valsartan effect on the outcomes in patients with heart failure is not exclusively related to angiotensin receptor antagonism and neprilysin inhibition, but rather in the fact that it has a direct effect on the extracellular matrix turnover, decreasing significantly the precursors of collagen synthesis in patients with heart failure with reduced ejection fraction and an active fibrotic process.
This investigation has not received specific aid from public sector agencies, the commercial sector or non-profit entities.
Conflicts of interestNone.
We thank to the multidisciplinary team of Heart Failure Unit of DIME Clínica Neurocardiovascular for their decisive and valuable contribution in the realization of this project.
Abbreviations: ECM, extracellular matrix; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; PINP, procollagen type I amino-terminal peptide; PIIINP, procollagen type III amino-terminal peptide.