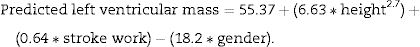Inappropriate left ventricular mass (iLVM) in patients with aortic stenosis (AS) was associated with unfavorable cardiovascular outcome. Our aim was to study the prevalence and characteristics of iLVM in patients with severe AS.
MethodsThose patients with severe AS (valve area <1cm2) in our Echocardiography Laboratory between January 2016 and January 2018 were prospectively included. iLVM was diagnosed when the measured left ventricular mass exceeded by 28% the expected value predicted from height, sex and stroke work. Patients with significant left ventricular dysfunction (left ventricular ejection fraction <40%) were excluded.
ResultsNinety patients were included (mean age 76±9 years, 55% women), 83% with iLVM. Patients with iLVM had lower indexed aortic valve area (0.39±0.08 vs 0.48±0.06cm2/m2, P<.001), time–velocity integral ratio (0.22±0.04 vs 0.27±0.04; P<.001), indexed stroke volume (39±9 vs 51±13mL/m2, P<.001), higher acceleration time (113±18 vs 88±22ms, P=.001), acceleration time/ejection time ratio (0.37±0.05 vs 0.28±0.07; P<.001) and valvuloarterial impedance (4.8±1.1 vs 4.2±1.0mmHg/mL/m2, P=.04), whilst we did not find differences in mean gradient (48±11 vs 18±12mmHg; P=.96) or peak velocity (4.4±0.5 vs 4.4±0.5m/s; P=.99). Left ventricular ejection fraction, acceleration time to ejection time ratio, indexed systolic volume and valvuloarterial impedance were the only determining variables of iLVM in multivariable analysis.
ConclusionsInappropriate left ventricular mass is a frequent finding in patients with severe AS, with echocardiographic characteristics similar to paradoxical low-flow low-gradient severe AS despite normal left ventricular ejection fraction.
La masa ventricular izquierda inapropiada (MVI) en pacientes con estenosis aórtica se ha asociado con un pronóstico cardiovascular desfavorable. Nuestro objetivo fue estudiar la prevalencia y características de la MVI en pacientes con estenosis aórtica grave.
MétodosSe incluyó prospectivamente a los pacientes diagnosticados de estenosis aórtica grave (área valvular <1cm2) en nuestro laboratorio de ecocardiografía entre enero de 2016 y enero de 2018. Se diagnosticó MVI cuando la masa ventricular izquierda estimada excedía un 28% del valor espera en función del sexo, altura y trabajo sistólico. Se excluyó a los pacientes con disfunción sistólica (fracción de eyección del ventrículo izquierdo <40%).
ResultadosSe incluyó a 90 pacientes (edad media 76±9 años, 55% mujeres), 83% con MVI. Los pacientes con MVI tenían menor área valvular indexada (0,39±0,08 frente a 0,48±0,06cm2/m2, p<0,001), ratio integral tiempo-velocidad (0,22±0,04 frente a 0,27±0,04; p<0,001), volumen sistólico indexado (0,22±0,04 frente a 0,27±0,04; p<0,001), mayor tiempo de aceleración (113±18 frente a 88±22ms, p=0,001), ratio tiempo de aceleración/tiempo de eyección (0,37±0,05 frente a 0,28±0,07; p<0,001) e impedancia valvuloarterial (4,8±1,1 frente a 4,20±1,0mmHg/ml/m2, p=0,04), aunque no se encontraron diferencias en gradiente medio (48±11 frente a 18±12mmHg; p=0,96) ni velocidad máxima (4,4±0,5 frente a 4,4±0,5m/s; p=0,99). La fracción de eyección de ventrículo izquierdo, ratio tiempo de aceleración/tiempo de eyección, el volumen sistólico indexado y la impedancia valvuloarterial fueron las únicas variables determinantes de MVI en el análisis multivariante.
ConclusionesLa MVI es un hallazgo frecuente en pacientes con estenosis aórtica grave, con características ecocardiográficas similares a la estenosis aórtica grave de bajo fujo-bajo gradiente a pesar de una fracción de eyección de ventrículo izquierdo normal.
In aortic stenosis (AS), left ventricular afterload is thought to initially increase wall stress and stimulate parallel myocyte growth leading to left ventricular hypertrophy,1,2 representing the effort of the cardiovascular system to keep wall stress close to normal values.3 Over time, left ventricular hypertrophy becomes an inconvenient adaptation mechanism that leads to impairment left ventricular performance.4
An excessive growth of left ventricular mass, a condition called “inappropriate left ventricular mass (iLVM)” was defined in arterial hypertension,5 and AS.6 It was associated with an unfavorable cardiovascular outcomes in arterial hypertension,7,8 and in AS.9
Our aim was to study the prevalence and characteristics of iLVM in patients with severe AS.
MethodsWe prospectively included 90 patients who attended our Echocardiography Laboratory between January 2016 and January 2018 with severe native valvular AS (aortic valve area <1cm2). The exclusion criteria were: age under 18 years old, suboptimal acoustic window, concomitant moderate or severe aortic regurgitation, moderate or severe mitral or tricuspid valvular disease, subvalvular o supravalvular aortic stenosis (defined as velocity higher than 1.5m/s), and ascending thoracic aorta diameter less than 25mm. We also excluded 2 patients with significant left ventricular dysfunction (left ventricular ejection fraction <40%).
The study protocol was approved by the Ethics Committee of Cadiz province. All the participants gave their consent to participate in the study.
Echocardiographic examinationTwo-dimensional transthoracic echocardiographic and Doppler studies were obtained with clinical ultrasound machines equipped with 2.5–3.5MHz transducers (iE33 Phillips Medical Systems, The Best, The Netherlands). All tests were conducted by 2 experienced sonographers. Blood pressure and heart rate were measured at the time of the echocardiographic evaluation.
Left ventricular chamber dimension and wall thicknesses were measured, and then left ventricular mass was calculated according to the American Society of Echocardiography guidelines.10 Left ventricular hypertrophy was defined as indexed left ventricular mass of 95g/m2 or greater for women and 115g/m2 or greater for men.10 The relative wall thickness was calculated as posterior wall thickness/left ventricular diastolic radius ratio independently of the presence of left ventricular hypertrophy and indicated concentric left ventricular geometry if 0.42 or greater.
An excess of left ventricular mass was assessed as the ratio between the observed and predicted value. Left ventricular mass was predicted from stroke work, gender and body size (as height (m) to the 2.7 power) by the following equation11:
Stroke work was estimated from brachial systolic blood pressure plus continuous wave Doppler transaortic peak gradient times stroke volume and converted to grammeters by multiplying by 0.0144. Gender was assigned the value of 1 for men and 2 for women.
iLVM was defined as when the ratio between the observed and predicted left ventricular mass was greater than 1.28.9
Parasternal long axis view with zoom was used for measuring the aortic annulus diameter in early systole. Using the pulsed Doppler in the left ventricular outflow tract, placing the sample volume 1cm below the aortic valve, the time–velocity integral was obtained. Stroke volume was then calculated assuming a circular shape of the left ventricular outflow tract. Continuous wave Doppler recording of flow through the valve was performed from all views to record maximal instantaneous and mean pressure gradients across the aortic valve.
Effective orifice area (EOA) was calculated using the continuity equation. An indexed EOA was estimated as EOA/body surface area. Mean transvalvular pressure gradient was obtained using of the modified Bernoulli equation. A Doppler velocity index, a simplification of the continuity equation, was calculated as time–velocity integral of left ventricular outflow tract/time–velocity integral of aortic jet. We also estimated the systolic work loss as (100*aortic mean gradient) divided by (aortic mean gradient+systolic blood pressure).12 As a measure of global left ventricular afterload, we calculated the valvuloarterial impedance as (systolic arterial pressure+mean gradient) divided by stroke volume index.13
Ejection time, acceleration time and acceleration time/ejection time ratio were also measured using the velocity curve from the continuous wave Doppler recording, as previously reported.14,15
All measurements represent an average of 3 cardiac cycles for patients in sinus rhythm and at least 6 cycles if the patient was in a rhythm other than than sinus rhythm. In any case, the estimation of extrasystolic beat was always avoided. Doppler recordings were performed at a sweep speed of 150mm/s.
In patients with severe AS, discordance was defined as an EOA <1cm2 and mean gradientmmHg. A “paradoxical” low flow-low gradient severe AS16 was defined as a EOA smaller than 1.0cm2, mean gradient less than 40mmHg, left ventricular ejection fraction above 50% and stroke volume index ≤35mL/m2.
We also analyzed diastolic function according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging guidelines.17
OutcomeThe primary endpoint was combined (cardiac death or aortic valve replacement). Outcomes data were obtained from patient visits or records, telephone interviews or death certificate when applicable.
Statistical analysisContinuous variables are presented as mean±standard deviation and compared using the unpaired Student t test. Categorical variables were expressed as percentages and compared using chi-square analysis or Fisher's exact test. The comparison between different echocardiographic parameters and iLVM was calculated by Pearson correlation.
Multiple linear regression models were developed to study the relationship between iLMV and the variables that were significant in univariate analysis.
Differences were considered significant at P<.05. For data analysis, the statistical program SPSS version 20.0 (SPSS Inc., Chicago, USA) was used.
ResultsNinety patients were included (mean age 76.5±8.7, 55% women): 75 patients (83%) with iLVM and 15 with “normal” left ventricular hypertrophy. These patients had a high prevalence of comorbidities: 78% arterial hypertension, 40% diabetes, 20% creatinine clearance less than 30mL/m2.
Basal characteristics comparison between patients with or without iLVM is summarized in Table 1, without any significant differences.
Basal characteristics in patients with and without iLVM.
| Variable | iLVM (n=75) | No iLVM (n=15) | P |
|---|---|---|---|
| Sex (women) | 40 (53%) | 10 (66%) | .34 |
| Age | 78.4±7.1 | 78.7±7.7 | .87 |
| BMI (kg/m2) | 29.3±6.0 | 30.2±5.8 | .60 |
| Arterial hypertension | 62 (82%) | 8 (61%) | .13 |
| Diabetes | 30 (40%) | 6 (46%) | .68 |
| Degenerative etiology | 68 (91%) | 14 (93%) | .86 |
| Symptoms | 66 (88%) | 12 (80%) | .65 |
| Cr clearance <30mL/min/1.73m2 | 15 (20%) | 3 (20%) | .99 |
BMI, body mass index; Cr, creatinine; iLVM, inappropriate left ventricular mass.
Patients with iLVM had lower EAO, Doppler velocity index ratio, indexed stroke volume, and higher acceleration time/ejection time ratio and valvuloarterial impedance, as you can see in Table 2. However, no significant differences in mean gradient or peak velocity were found. We only had 4 patients (4%) with paradoxical severe AS, all in iLVM group.
Echocardiographic differences between patients with and without iLVM.
| Variable | iLVM (n=75) | No iLVM (n=15) | P |
|---|---|---|---|
| Indexed LVM | 143.3±34.8 | 104.0±25.4 | <.001 |
| EOA (cm2) | 0.70±0.12 | 0.86±0.11 | <.001 |
| Indexed EOA (cm2/m2) | 0.39±0.08 | 0.48±0.06 | <.001 |
| Mean gradient (mmHg) | 48.3±11.3 | 48.1±12.6 | .96 |
| Peak velocity (m/s) | 4.38±0.52 | 4.38±0.59 | .99 |
| TVI ratio | 0.22±0.04 | 0.27±0.04 | <.001 |
| SWL | 26.6±5.9 | 23.8±5.3 | .07 |
| Zva (mmHg/mL/m2) | 4.8±1.1 | 4.2±1.0 | .04 |
| AT (ms) | 113±18 | 88±22 | <.001 |
| ET (ms) | 314±30 | 318±25 | .69 |
| AT/ET ratio | 0.37±0.05 | 0.28±0.07 | <.001 |
| Heart rate (bpm) | 71±14 | 72±8 | .82 |
| Indexed stroke volume (mL/m2) | 39.4±9.4 | 50.9±13.3 | <.001 |
| Paradoxical AS | 4 (5%) | 0 (0%) | 0.36 |
| LVEDD (mm) | 45.9±7.1 | 40.7±6.4 | 0.01 |
| Relative wall thickness | 0.52±0.12 | 0.45±0.11 | 0.04 |
AT, acceleration time; bpm, beats per minute; ET, ejection time; EOA, effective orifice area; iLVM, inappropriate left ventricular mass; LVEDD, left ventricular end-diastolic diameter; LVM, left ventricular mass; SWL, stroke work loss; TVI, time velocity integral; Zva, valvulo-arterial impedance.
Different parameters of left ventricular diastolic function were analyzed. Although E/e′ ratio was the only variable that was significantly higher in iLVM patients, we observed a greater diastolic dysfunction in this group (Table 3).
Diastolic function parameters in both groups.
| Variable | iLVM (n=75) | No iLVM (n=15) | P |
|---|---|---|---|
| E wave (m/s) | 0.92±0.21 | 0.83±0.24 | .14 |
| A wave (m/s) | 1.04±0.24 | 1.05±0.30 | .85 |
| Lateral E′ (cm/s) | 7.15±1.66 | 7.85±1.91 | .14 |
| Medial E′ (cm/s) | 5.92±1.10 | 6.45±1.77 | .11 |
| LA volume (mL/m2) | 40.0±10.2 | 38.9±14.0 | .66 |
| Mean E′ (cm/s) | 6.63±1.17 | 7.18±1.80 | .10 |
| E/E′ | 15.3±2.9 | 12.6±3.9 | .04 |
| Normal/indeterminate/dysfunction | 0/38/37 | 1/10/4 | .04 |
iLVM, inappropriate left ventricular mass.
Univariate correlations between inappropriate left ventricular mass and clinical and echocardiographic variables are shown in Table 4 and Fig. 1. We observed again that iLVM was related with flow-independent AS severity parameters (EOA, indexed EOA, velocity Doppler index, acceleration time/ejection time ratio) and an inverse relationship with indexed stroke volume, but not with flow-dependent variables (mean gradient and peak velocity).
Pearson correlation between inappropriate left ventricular mass and clinical and echocardiographic variables.
| Variable | Pearson coefficient (r) | P |
|---|---|---|
| Systolic arterial pressure (mmHg) | −0.52 | <.001 |
| LVEF (%) | −0.40 | <.001 |
| Peak velocity (m/s) | −0.14 | .18 |
| Mean gradient (mmHg) | −0.13 | .21 |
| EOA (cm2) | −0.43 | <.001 |
| Indexed EOA (cm2/m2) | −0.40 | <.001 |
| AT/ET ratio | 0.47 | <.001 |
| Age (years) | 0.07 | .51 |
| BMI (kg/m2) | 0.06 | .56 |
| Indexed systolic volume (mL/m2) | −0.46 | <.001 |
| Doppler velocity index | −0.56 | <.001 |
| Zva (mmHg/mL/m2) | 0.16 | .13 |
AT/ET, acceleration time/ejection time; BMI, body mass index; EOA, effective orifice area; LVEF, left ventricular ejection fraction; Zva, valvulo-arterial impedance.
Multiple linear regression analysis was performed to evaluate the variables that determine iLVM. Results of the model are represented in Table 5. This model had a R2 of 0.38, being acceleration time to ejection time ratio, left ventricular ejection fraction, indexed systolic volume and valvuloarterial impedance the significant determining variables of iLVM.
Multiple linear regression model for iLVM (R2=38%).
| Variable | Coefficient (standardized regression coefficient) | P |
|---|---|---|
| Systolic arterial pressure (mmHg) | 0.23 (0.16) | .17 |
| AT/ET ratio | 192.8 (0.35) | .002 |
| LVEF (%) | −0.72 (−0.23) | .04 |
| EOA (cm2) | 5.74 (0.022) | .87 |
| Indexed systolic volume (mL/m2) | −0.24 (−0.23) | .03 |
| Zva (mmHg/mL/m2) | −23.16 (−0.32) | .02 |
| Doppler velocity index | −10.0 (−0.14) | .21 |
AT/ET, acceleration time/ejection time; LVEF, left ventricular ejection fraction; EOA, effective orifice area; Zav, valvuloarterial impedance.
Follow-up was achieved in 86 patients (95%), with a mean follow-up time of 188±101 days, without significant differences between both groups (P=.72). During the follow-up, 53 patients reached the combined end-point (37 patients with aortic valve replacement and 16 patients died from cardiovascular cause). The combined-end point was more frequent in patients with iLVM (66% vs 40%, P=.10) but we failed to find significant differences (Table 6).
DiscussionThe adjustment of left ventricular mass for left ventricular performance allows us to evaluate the appropriateness of left ventricular hypertrophy in patients with chronic left ventricular overload. Values of sex-specific left ventricular mass exceeding those expected for body size and stroke work, namely ‘inappropriate LV mass’ (iLVM), have been considered as a redundant and harmful biological response to increased left ventricular load.11 iLVM have been especially studied in arterial hypertension.7,8,11,18
The main findings of our study were: a) The prevalence of iLVM in patients with severe AS is high, and b) patients with iLVM had lower stroke volume, so they could not achieve higher transvalvular gradients despite lower aortic valve area.
Our sample had patients with more severe AS than other studies,9 taking into account echocardiographic parameters and symptomatic status. This could be the explanation of the higher prevalence of iLVM found in our study.
Although left ventricular remodeling is considered a compensatory mechanism in AS,19 as the left ventricle hypertrophies, myocardial oxygen demand increases.9 Besides, coronary flow reserve is also reduced because microvascular dysfunction, low coronary flow perfusion pressure, increased extravascular compressive forces, and reduced diastolic perfusion time.20 These factors induce interstitial fibrosis, that is the primary cause of diastolic dysfunction in AS.21
To our knowledge, this is the first study that demonstrates that AS patients with iLVM has greater diastolic dysfunction. Some studies have demonstrated that with worsening diastolic dysfunction, end-diastolic pressure rises, reducing coronary artery pressure and increasing ischemia, further perpetuating fibrosis.22 Besides, myocardial fibrosis in AS has been shown as a poor prognostic marker.23,24 This could be the explanation of the poorer prognosis of AS patients with iLVM in other studies.9
Paradoxical low-flow low-gradient severe AS in the setting of preserved left ventricular ejection fraction is increasingly being recognized.25–30 This entity is characterized by more pronounced left ventricular hypertrophy and diastolic dysfunction,31 showing similar or worse EOA but their gradients are lower because of the characteristic low stroke volume of this disease.32,33 Our study is the first that shows that patients with iLVM shares similar characteristics with paradoxical low-flow low-gradient severe AS. Probably, inappropriate hypertrophy and the corresponding diastolic dysfunction could be the pathophysiological pathway of paradoxical severe AS.
Low stroke volume has been shown as a poor prognostic factor in AS,34 independently of left ventricular ejection fraction. We found an independent correlation between indexed stroke volume and iLVM. Some authors have found a impaired longitudinal left ventricular function by speckle-tracking despite normal left ventricular ejection fraction,35,36 and LV fibrosis has been implicated as the underlying pathologic process.37 This could explain the association between iLVM and low stroke volume of our study.
Arterial hypertension contributes to left ventricular remodeling,1,5,6 but we decided to not exclude hypertensive patients because arterial hypertension and AS are frequent comorbidities. Our results demonstrate the importance of global afterload in left ventricle remodeling, since valvuloarterial impedance was an independent factor in iLVM patients. Hachicha et al.32 showed a significant correlation between higher valvuloarterial impedance and paradoxical low-flow low-gradient severe AS, with similar findings to our results.
LimitationsThe main limitation of the study was the small sample size that makes difficult to find other statistically significant differences. So we only found four patients with paradoxical severe low-flow low-gradient AS, although our prevalence was similar to other recent studies.34 We could probably find a significant association between iLVM and paradoxical severe AS if we increased the sample.
Although left ventricle mass determined by cardiac magnetic resonance could be more accurate and precise,38 we preferred using echocardiography because of its versatility and availability. Besides, left ventricular mass assessed by echocardiography has an acceptable correlation with cardiac magnetic resonance,39 and has been shown as an outcome predictor.40
Unfortunately, we only performed speckle tracking in 6 patients, so we could not find a possible correlation between subclinical systolic dysfunction and iLVM.
Although theoretically indexed EOA could have advantages assessing AS severity, we preferred using EOA without indexation because indexed EAO increase echocardiographic inconsistencies with respect to gradients.41
Although other authors have demonstrated the prognostic value of iLVM,9 we failed to find significant differences between groups because of the small sample size.
ConclusionsIn conclusion, iLVM is a frequent finding in patients with severe AS, and it could be the pathophysiological pathway that explain paradoxical low-flow low-gradient severe AS despite normal left ventricular ejection fraction.
- -
An excessive growth of left ventricular mass, a condition called ‘inappropriate left ventricular mass’ was associated with unfavorable cardiovascular outcomes in aortic stenosis.
- -
Inappropriate left ventricular mass was present in 83% of patients with severe aortic stenosis.
- -
This condition was associated with lower indexed aortic valve area, Doppler velocity index and higher acceleration time to ejection time ratio.
- -
Characteristics of these patients were similar to paradoxical low flow-low gradient severe aortic stenosis.
No conflict of interests.
Abbreviations: AS, aortic stenosis; iLVM, inappropriate left ventricular mass.