Bleeding and ischemic risk after acute coronary syndrome (ACS) is not uniform over time. Our objective was to determine the differences in post-ACS ischemic-hemorrhagic balance between diabetic and non-diabetic patients treated with dual antiplatelet therapy with prasugrel or ticagrelor.
MethodsFrom 4424 patients enrolled in the RENAMI registry, 1323 (29.9%) had diabetes. The average daily ischemic risk (ADIR) and average daily bleeding risk (ADBR) were defined as the total number of events in that specific time interval divided by the total number of patient-days of follow-up. The ischemic-hemorrhagic balance was calculated as the difference between ADIR and ADBR.
ResultsIschemic and hemorrhagic risks were high in the first 30 days after ACS (ADIR 0.014% in diabetics and 0.012% in non-diabetics; ADBR 0.012% in diabetic and 0.008% in non-diabetic patients) and decreased later. In diabetic patients, the risk of reinfarction is higher than the bleeding risk, especially in the first month (ADIR minus ADBR: +0.002%) and after 6 months (ADIR minus ADBR: 0.007%). However, in non-diabetic patients, the risk of bleeding is higher than the risk of reinfarction, mainly between third and ninth months (ADIR minus ADBR: −0.001%). After propensity score matching, differences found in ischemic-hemorrhagic balance were consistent.
ConclusionsThe incidence of both hemorrhagic and ischemic events is higher in the first 30 days. During the first year, in diabetic patients there is a predominance of reinfarction against bleeding. In contrast, in non-diabetic patients, the risk of bleeding is higher than the risk of reinfarction, with the exception of the first 2 and last month.
El equilibrio isquémico-hemorrágico tras un síndrome coronario agudo (SCA) varía durante el seguimiento. Nuestro objetivo es determinar las diferencias en el equilibrio isquémico-hemorrágico en pacientes diabéticos y no diabéticos tratados con ticagrelor o prasugrel tras un SCA.
MétodosDe 4.424 pacientes incluidos en el registro RENAMI, 1.323 (29,9%) eran diabéticos. La tasa media diaria de eventos isquémicos (TMEI) y hemorrágicos (TMEH) se definió como el número total de eventos en dicho periodo de tiempo dividido por el total de pacientes-día en seguimiento. El equilibrio isquémico-hemorrágico se calculó como la diferencia entre TMEI y TMEH.
ResultadosEl riesgo de eventos isquémicos y hemorrágicos fue elevado en los 30 primeros días tras un SCA (TMEI 0,014% en diabéticos y 0,012% en no diabéticos; TMEH 0,012% en diabéticos y 0,008% en no diabéticos) y disminuyó posteriormente. En los pacientes diabéticos, el riesgo isquémico era mayor que el hemorrágico, especialmente en el primer mes (TMEI menos TMEH: 0,002%) y a partir del sexto mes (TMEI menos TMEH: 0,007%). Sin embargo, en pacientes no diabéticos, el riesgo hemorrágico supera al riesgo isquémico, principalmente entre el tercer y noveno mes de seguimiento (TMEI menos TMEH: -0.001%). Las diferencias se mantuvieron tras el análisis de propensión.
ConclusionesLa incidencia de eventos isquémicos y hemorrágicos es mayor en los primeros 30 días tras un SCA. En los pacientes diabéticos predomina el riesgo de reinfarto sobre el hemorrágico durante el primer año de seguimiento. Por el contrario, en pacientes no diabéticos, el riesgo hemorrágico supera al riesgo isquémico, con excepción de los 2 primeros y el último mes de seguimiento.
Diabetes mellitus (DM), regardless of its association with other cardiovascular risk factors, confers a higher and independent risk of developing atherosclerotic disease, the latter accounting for approximately 70% of the causes of mortality in DM patients. In addition to the classic atherogenic and atherothrombotic risk factors, such as age, smoking or hypertension, other specific DM factors are added, such as endothelial dysfunction, alteration of hemostatic processes (platelet activation, coagulation, and fibrinolysis) or diabetic dyslipidemia. In patients with acute coronary syndrome (ACS), DM has been associated with a worse prognosis, with a marked increase in reinfarctions and mortality. For this reason, more aggressive therapeutic strategies are usually used in DM patients. In this way, the recently published European Society of Cardiology Guidelines for the management of ACS in patients presenting without persistent ST-elevation propose DM requiring medication as a risk criterion for extended antithrombotic treatment beyond 12 months after ACS.1 However, it is also known that DM increases the risk of bleeding, as a result of microangiopathy and endothelial damage secondary to hyperglycemia. To date, no study has balanced post-ACS ischemic-hemorrhagic risk in DM and non-DM while receiving dual antiplatelet therapy (DAPT). The aim of this study was to characterize the differences in ischemic and hemorrhagic balance between DM and non-DM patients treated with DAPT with prasugrel or ticagrelor after ACS.
MethodsThis study uses data from the RENAMI (new antiplatelet therapy in patients with acute myocardial infarction registry) registry, a retrospective, observational, multicenter, and international registry made up of a total of 4424 patients, in which 12 centers of 6 European countries (Spain, Italy, Switzerland, Greece, Serbia, United Kingdom) voluntarily participated. RENAMI included patients aged≥18 years with ACS (non-ST segment elevation ACS, ST segment elevation ACS, and unstable angina) who underwent coronary angiography and percutaneous coronary intervention (PCI) and were treated with DAPT using aspirin and ticagrelor or prasugrel in any period between January 2012 and January 2016. The registry and all subsequent derived studies have been developed in accordance with the Declaration of Helsinki. Due to the large number of patients and that many of them had already died at the time of data collection, RENAMI was approved by the local ethics committees without the requirement for patients’ informed consent. Given its retrospective nature, diagnostic and therapeutic procedures were performed according to the preferences of each center and cardiologist. Patients were classified as diabetic based on the definition of DM proposed by the American Diabetes Association at the time of hospital discharge, to also include those diagnosed during admission.
The aim of this study was to determine the impact of DM on the post-ACS net ischemic-hemorrhagic balance in patients receiving DAPT with ticagrelor or prasugrel; defined as the difference between ischemic average daily rates (ADIR) and bleeding average daily rates (ADBR).
Data on follow-up events were obtained from hospital and administrative clinical data records. Bleeding events were defined as any primary Bleeding Academic Research Consortium 3–5 bleeding (major bleeding). As ischemic events, spontaneous acute myocardial infarction (AMI; defined as the presence of elevation of markers of myocardial damage above the upper limit of normal in combination with angina symptoms or electrocardiographic alterations compatible with myocardial ischemia) were included.
This study only considered the first ischemic or hemorrhagic event that took place during the period of treatment with DAPT. As the registries analyzed in this study take into consideration only single events, patients who had an event were excluded from the population at risk thereafter. Follow-up was censored in the event of death, ischemic or hemorrhagic episodes, suspension/withdrawal of DAPT or end of follow-up in the local clinical registry, whichever occurred first.
Continuous variables are presented as mean (standard deviation, SD). Discrete variables are expressed as counts and percentages and compared with Pearson's Chi-squared test. Continuous variables were compared with Student's t test.
Like in previous studies,2 instantaneous daily bleeding and ischemic rates were calculated dividing the number of events occurring in a specific day post-PCI for ACS by the number of exposed patients on the same day. ADIR and ADBR were defined as the total number of events in that specific time interval divided by the total number of patient-days of follow-up, that is, the total number minus loss at follow-up, deaths, and people who already had an event.
The difference between ADIR and ADBR was calculated to assess the net ischemic-hemorrhagic balance and detect the presence of a potential excess of ischemic/hemorrhagic events in any given period or in a specific subgroup. Positive differences indicate excess ischemic risk, while negative differences indicate excess bleeding risk. After calculation of daily risks, paired t test was applied to verify if there was a significant difference in term of ischemia/bleeding during the various time frames and subgroups. Estimated mean daily event rate is represented by fractional polynomials and the difference between ADIR and ADBR is represented by quadratic regression.
Differences in death outcomes between DM and no-DM patients were assessed using multivariate Cox proportional hazards regression models, with robust variance estimators to account for inter-hospital variability in clinical practice. The proportional hazards assumption was tested using the Schoenfeld residual test, which showed no nonproportionality. Hazard ratios (HR), with their 95% confidence intervals (95%CI), were obtained. Ischemic and bleeding endpoints were assessed by Fine and Gray competing-risk multivariate regression, considering death and non-fatal events (reinfarction and bleeding) as competing risks. Results were reported as sub-distribution HR (sHR) and 95%CI. The goodness of fit was investigated using a Gronnesby and Borgan test and the discriminative performance of the survival models was evaluated by Harrell C-index. A univariate Fine and Gray regression model was used to assess predictors of ischemic and hemorrhagic events out of the total sample.
We also performed a propensity score matching analysis to obtain two comparable groups (DM and non-DM) in terms of baseline characteristics, clinical presentation, and treatment strategies. We calculated the propensity score by multivariate logistic regression analyses, based on all the baseline covariates listed in Table 1.
Baseline features of patients after propensity score matching.
| Baseline feature | DM(n=924) | Non-DM(n=924) | SD |
|---|---|---|---|
| Duration of DAPT, months | 12.25 (3.77) | 12.25 (3.76) | −0.001 |
| Duration of DAPT<6 months | 52 (5.6) | 50 (5.4) | 0.009 |
| Age, years | 63.3 (11.4) | 63.7 (10.8) | −0.036 |
| Female sex | 202 (21.9) | 190 (20.6) | 0.030 |
| Smoking | |||
| Past (> 6 months) | 234 (25.3) | 239 (25.9) | −0.012 |
| Active (< 6 months) | 174 (18.8) | 163 (17.6) | 0.033 |
| Body mass index, kg/m2 | 28.1 (3.9) | 28.1 (4.5) | 0.009 |
| Hypertension | 632 (68.4) | 659 (71.3) | −0.061 |
| Dyslipidemia | 537 (58.1) | 534 (57.8) | 0.007 |
| Peripheral artery disease | 30 (3.3) | 24 (2.6) | 0.040 |
| Prior myocardial infarction | 183 (19.8) | 175 (18.9) | 0.020 |
| Prior stroke | 56 (6.1) | 52 (5.6) | 0.014 |
| Prior bleeding | 24 (2.6) | 25 (2.7) | −0.006 |
| Prior cancer | 48 (5.2) | 47 (5.1) | 0.005 |
| Unstable angina | 101 (10.9) | 114 (12.3) | −0.048 |
| STEMI | 491 (53.1) | 505 (54.7) | −0.030 |
| Creatinine at admission, mg/dL | 1.0 (0.5) | 1.0 (0.5) | 0.085 |
| Hemoglobin at admission, g/dL | 14.0 (1.3) | 14.1 (1.3) | −0.050 |
| LVEF<40% | 104 (11.3) | 105 (11.4) | −0.003 |
| Multivessel CAD | 471 (51.0) | 465 (50.3) | 0.013 |
| Drug eluting stent | 626 (67.7) | 611 (66.1) | 0.037 |
| Anticoagulation | 15 (1.6) | 14 (1.5) | 0.008 |
| Antiplatelet therapy | 0.048 | ||
| Ticagrelor | 530 (57.4) | 551 (59.6) | |
| Prasugrel | 394 (42.6) | 373 (40.4) | |
| Betablockers | 882 (95.5) | 872 (94.4) | 0.060 |
| ACEI/ARB | 858 (92.9) | 849 (91.9) | 0.044 |
| Statins | 913 (98.8) | 912 (98.7) | 0.011 |
Continuous variables are presented as mean (SD); discrete variables are expressed as n (%). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; LVEF, left ventricular ejection fraction; SD, standardized deviation; STEMI, ST-segment elevation myocardial infarction.
Statistical analyzes were carried out with Stata/MP 15.0 and SPSS 24.0. Statistical significance was defined as a P value<.05.
ResultsA total of 4424 patients were evaluated, of which 29.9% (n=1323) were diabetic. The mean age of the total sample was 60.9 (SD 11.5) years, and 20.8% of the patients (n=920) were women. Main baseline characteristics are presented in Table 1 of the supplementary data. The DM population is younger and with a lower proportion of women compared to non-DM patients. Among diabetic patients, a higher prevalence of cardiovascular risk factors and comorbidities was found.
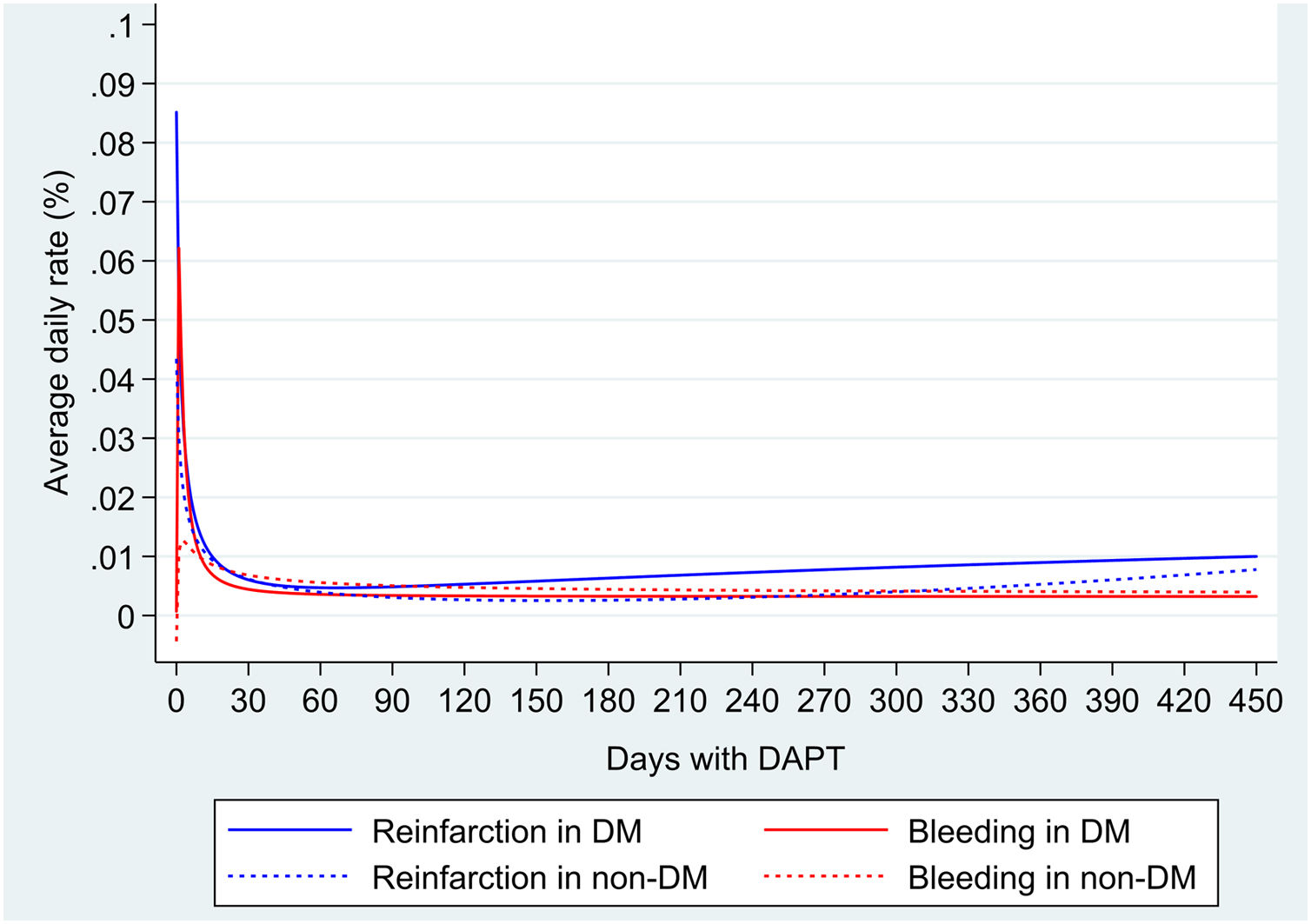
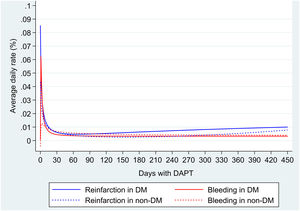
During a mean follow-up of 11.9 (SD 3.8) months (interquartile range, 12.0–12.4 months), a total of 67 deaths, 83 reinfarctions and 73 major bleeding were documented (Table 2 of the supplementary data). Mortality was significantly higher in DM population than in non-DM (HR 1.74; 95%CI, 1.07–2.83; P=.024). ADIR was higher in DM (2.77; 95%CI, 1.99–3.83 per 100 patient/years vs 1.57; 95%CI, 1.18–2.09 per 100 patient/years); ADBR were similar in both groups (1.69; 95%CI, 1.11–2.56 per 100 patient/years vs 1.7; 95%CI, 1.13–2.24 per 100 patient/years). Average daily risks in DM and non-DM patients were higher in the first 30 days, for both reinfarction (0.014% in DM vs 0.012% in non-DM) and major bleeding (0.012% in DM and 0.008% in non-DM) and subsequently decreased. After that, ischemic risk increases slightly from the second month in DM and the seventh month in non-DM, while the hemorrhagic risk remains stable or decreases during follow-up (Fig. 1).
Balancing ischemic and hemorrhagic risk, risk of reinfarction in DM patients is greater than bleeding risk, especially in the first month (ADIR minus ADBR: 0.002%) and from the sixth month (ADIR minus ADBR: 0.007%) after ACS. However, in non-DM patients, bleeding risk outweighs the risk of reinfarction, mainly between the second and the tenth month (ADIR minus ADBR: −0.001%).
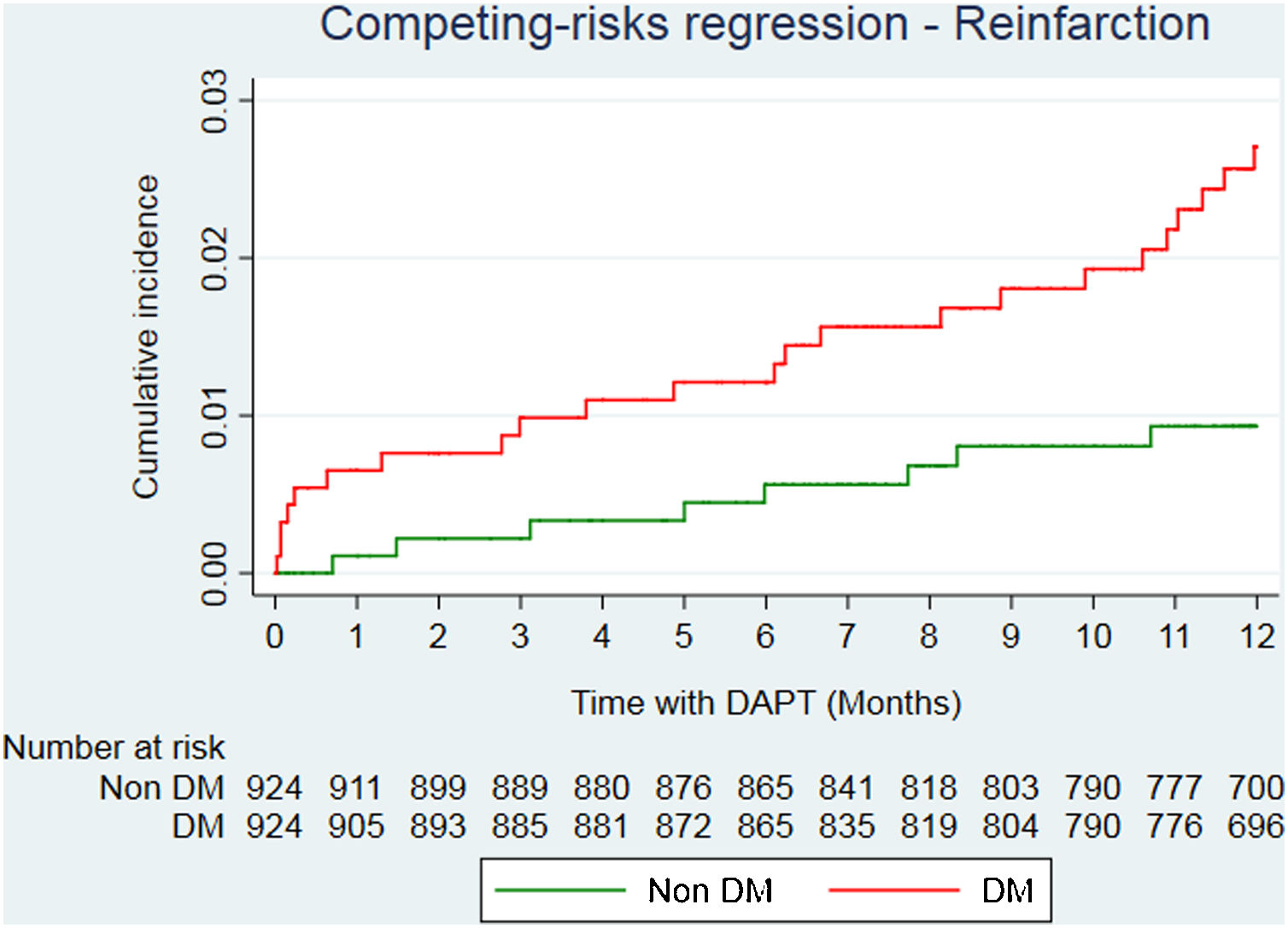
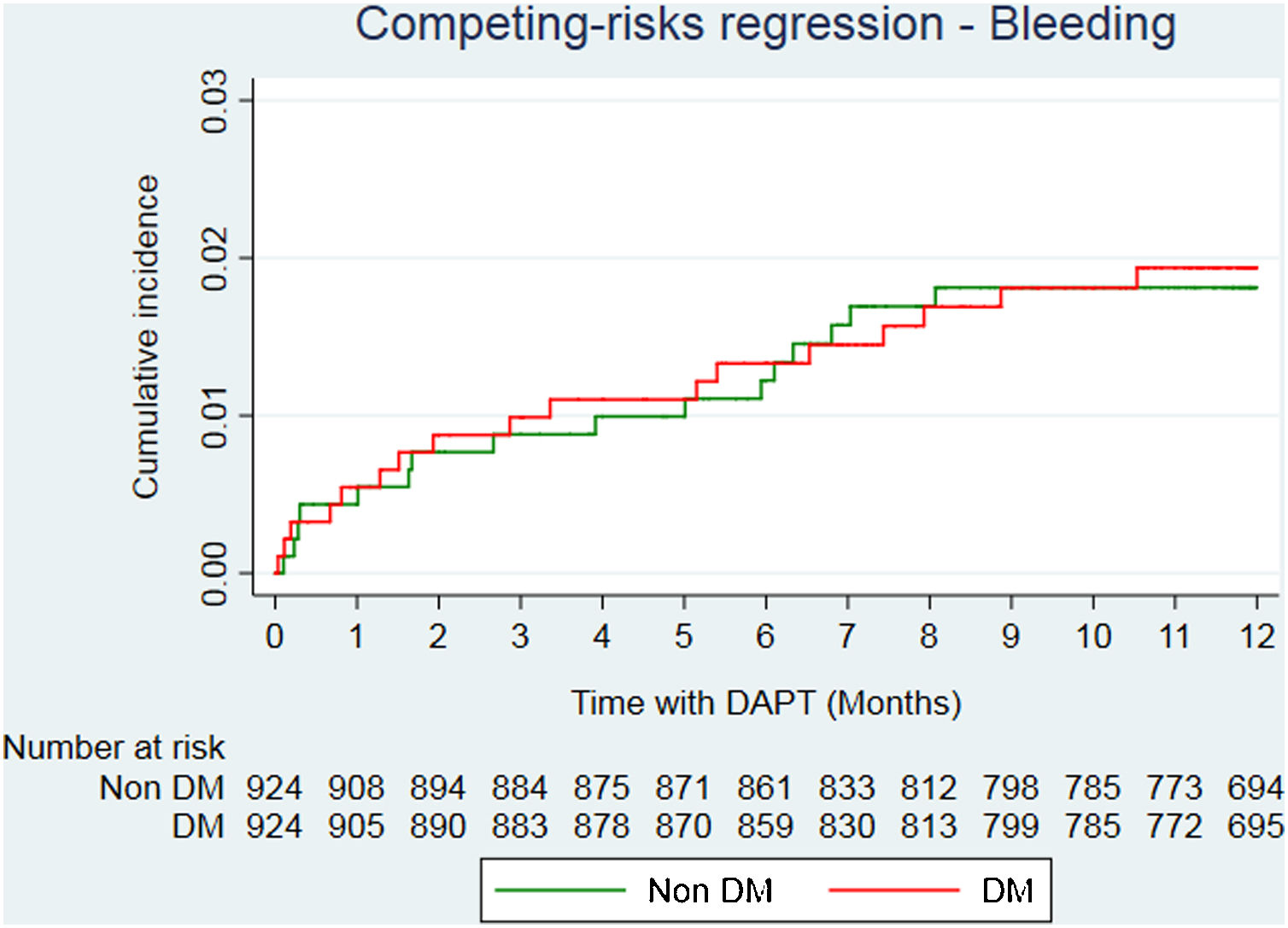
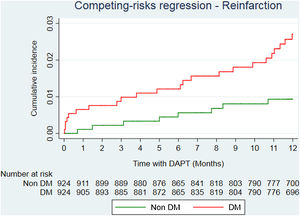
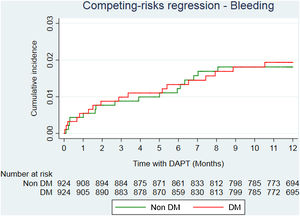
After propensity score matching analysis, 924 DM and 924 non-DM patients with similar baseline characteristics and treatment strategies were obtained (Table 1). A total of 36 deaths (23 in DM; 13 in non-DM); 39 reinfarctions (26 in DM; 13 in non-DM) and 34 major bleeding (17 in DM; 17 in non-DM) were recorded (Table 3 of the supplementary data). At the end of follow-up, DM was significantly associated with a higher risk of mortality (HR=2.05; 95%CI, 1.01–4.13; P=.046). Reinfarction rate in DM patients was almost double compared to non-DM patients (sHR=1.94; 95%CI, 0.96–3.89; P=0.064) (Fig. 2), with similar bleeding rates (sHR=1.14; 95%CI, 0.56–2.32; P=.720) (Fig. 3). Gronnesby and Borgan test and Harrel's C index show an adequate calibration and discrimination of survival models.
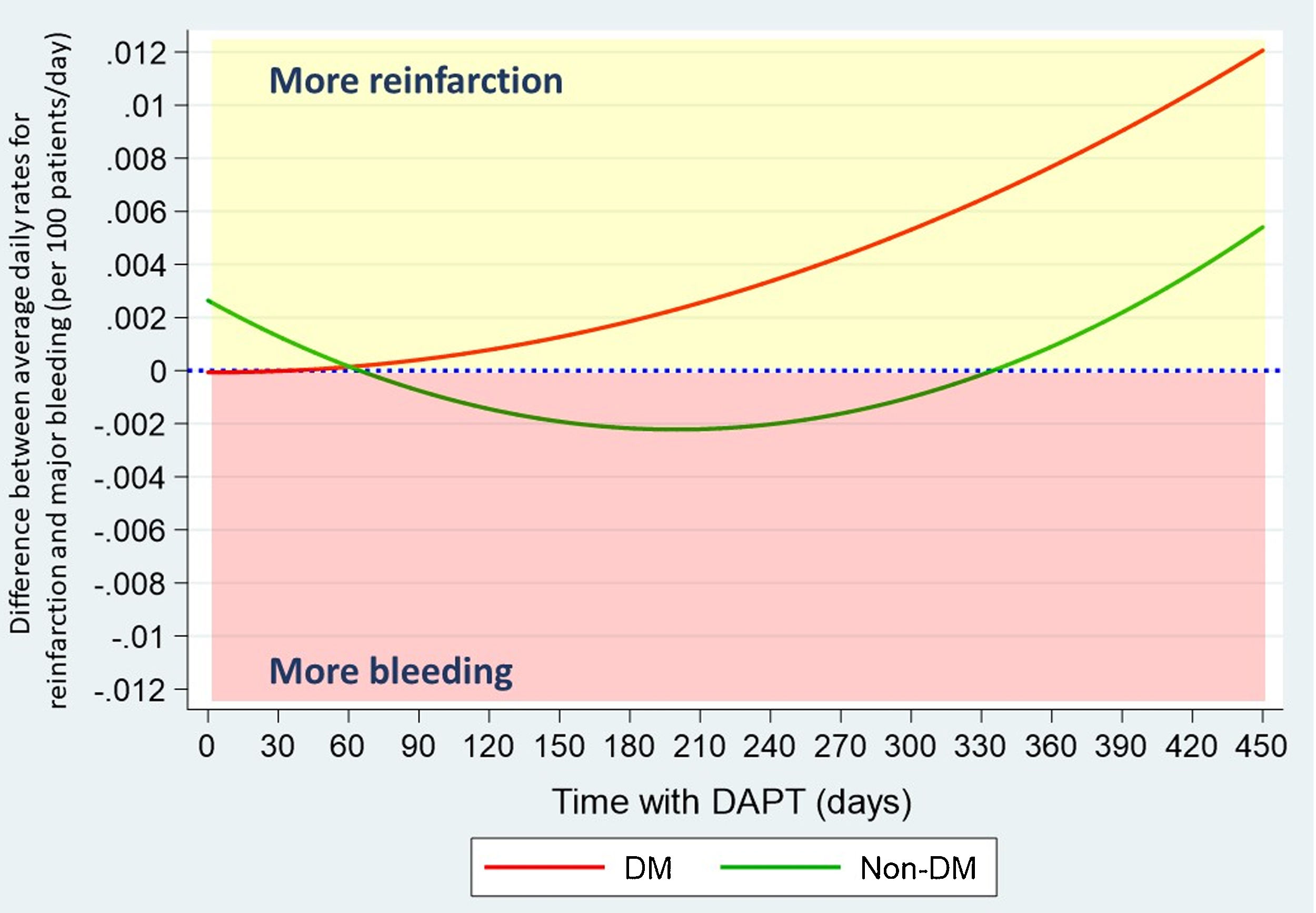
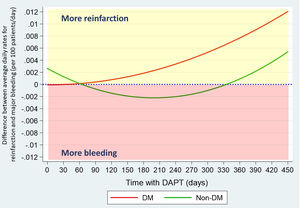
After propensity score matching, differences found in hemorrhagic ischemic balance were consistent. Ischemic risk predominates in DM patients, increasing progressively from the second month, while bleeding risk outweighs in non-DM, except for the first two and the last month of DAPT (Fig. 4).
Predictors of ischemic and hemorrhagic events are shown in Table 2. DM, dyslipidemia, peripheral arterial disease, previous myocardial infarction, ventricular dysfunction<40%) and multivessel disease were associated with higher risk of reinfarction. On the other hand, female sex, increasing age, arterial hypertension, previous bleeding and decreasing hemoglobin levels proved to be predictors of major bleeding. DM has not demonstrated to be a risk factor for bleeding in the present study.
Predictors of ischemic and hemorrhagic events.
| Baseline feature | Predictors of ischemic events (reinfarction) | Predictors of hemorrhagic events (major bleeding) | ||||
|---|---|---|---|---|---|---|
| sHR | 95%CI | P | sHR | 95%CI | P | |
| Age, per 1 year | 1.02 | 1.00–1.04 | .075 | 1.04 | 1.02–1.07 | <.001 |
| Female sex | 1.28 | 0.77–2.12 | .337 | 2.10 | 1.28–3.44 | .003 |
| Hypertension | 1.49 | 0.95–2.33 | .084 | 1.99 | 1.19–3.31 | .008 |
| Diabetes mellitus | 1.76 | 1.11–2.66 | .015 | 1.00 | 0.6–1.68 | .986 |
| Peripheral artery disease | 3.89 | 1.5–10.08 | .005 | 1.83 | 0.57–5.92 | .312 |
| Prior myocardial infarction | 2.02 | 1.25–3.26 | .004 | 0.87 | 0.44–1.69 | .675 |
| Prior bleeding | 0.57 | 0.08–4.09 | .575 | 3.37 | 1.36–8.33 | .009 |
| Hemoglobin, per 1 g/dL | 1.00 | 0.84–1.19 | .974 | 0.68 | 0.59–0.79 | <.001 |
| LVEF<40% | 2.40 | 1.39–4.15 | .002 | 0.58 | 0.21–1.59 | .288 |
| Multivessel CAD | 2.23 | 1.42–3.52 | .001 | 1.13 | 0.71–1.81 | .609 |
| Drug eluting stent | 0.80 | 0.51–1.26 | .333 | 0.55 | 0.34–0.87 | .011 |
| Prasugrel (vs ticagrelor) | 0.84 | 0.54–1.33 | .462 | 0.59 | 0.35–0.99 | .047 |
95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation myocardial infarction.
We present real-world data from daily clinical practice from a multicenter registry of DM and non-DM patients with ACS treated with ticagrelor and prasugrel after PCI. Several findings deserve special attention: (a) the ischemic-hemorrhagic balance of DM patients treated with DAPT with ticagrelor or prasugrel after ACS differs substantially compared to non-DM patients; (b) in patients treated with ticagrelor and prasugrel, mortality and reinfarction rates were higher in DM population than in non-DM. However, no significant differences were found in terms of major bleeding rates; (c) the rate of hemorrhagic and ischemic events in both DM and non-DM patients was higher in the first 30 days after ACS; (d) in non-DM patients, the bleeding risk exceeds the risk of reinfarction between the third and the eleventh month after ACS; and (e) on the contrary, in DM patients the risk of reinfarction exceeds the bleeding risk throughout the first year after ACS, with the exception of the first two months in which both risks are balanced.
There are multiple mechanisms that may explain the greater ischemic risk conferred by DM. Platelet structural abnormalities have been described in these patients, and also other phenomena like higher platelet turnover, overexpression of the P2Y12 pathway, endothelial dysfunction, metabolic disorders, inflammation, hyperglycemia or oxidative stress, that lead to increased platelet activity.3 Moreover, DM patients associate alterations in both coagulation and fibrinolysis pathways, with high concentrations of procoagulant factors and decreased concentrations of endogenous anticoagulants.3 All of this, together with a higher load of CVD risk factors and comorbidities, carries an increased risk of ischemic events in DM population.
Moreover, microvascular abnormalities and endothelial dysfunction also represent an increased risk of bleeding. Several studies have linked it not only with an increased risk of gastrointestinal4,5 and intracranial hemorrhage,6 but also with a greater morbidity and mortality of these events compared to non-DM patients.7–9 The evidence about the specific bleeding risk of the DM patient after a coronary event is heterogeneous, and largely depends on the time of evolution after it. In the CRUSADE Bleeding Score study, DM proved to be an independent predictor of risk of major in-hospital bleeding after non ST segment elevation ACS.10 In the PRECISE-DAPT score study, DM was not associated with a significant increase in major bleeding between 7 days and 12 months after PCI, neither in the derivation cohort nor in the validation cohort of the BernPCI registry (NCT02241291); however, it did in the validation cohort from the PLATO study.11 In the TRITON-TIMI-38 trial, DM was also associated with an increased risk of major bleeding, although without reaching statistical significance (P=.08).12,13 In the DAPT study, which explored predictors of bleeding in the period from 12 months to 30 months after PCI, DM was associated with an increased risk of ischemic but not hemorrhagic events.14 Finally, in a recent consensus document from the Academic Research Consortium for High Bleeding Risk (ARC-HBR), chronic bleeding diathesis (for instance, platelet dysfunction or clotting factor deficiencies) is proposed as a criterion for high bleeding risk, but DM by itself is not considered to be a risk factor.15
In our registry, with a mean follow-up period of approximately 12 months, we have found no significant difference in bleeding rates between DM and non-DM patients. It is possible that one of the reasons is the fact that only those patients treated with potent P2Y12 inhibitors (ticagrelor and prasugrel) were selected, excluding those with high bleeding risk (for example, those with a history of intracranial hemorrhage) in whom the first choice antiplatelet would be clopidogrel. Despite this, an interesting fact from our study is that the bleeding rate was much higher in the first 30 days after ACS, detecting approximately 50% of events in the first 3 months. After that, bleeding risk progressively decreases until it reaches its lowest rate at the end of follow-up. This finding is consistent with those presented in the PLATO and TRITON-TIMI-38 trials, which registered a higher incidence of bleeding in the first months, to subsequently decrease during follow-up.12,13,16,17 Reinfarction rates are also higher in the first 30 days but, on the contrary, they increase after the first 3 months and markedly after the eleventh month. Therefore, time elapsed after ACS seems highly relevant to correctly balance the ischemic and hemorrhagic risks and optimize antithrombotic treatment.
In non-DM patients, the bleeding risk seems to exceed the ischemic risk between the third and eleventh month after PCI. Considering this excessive risk of bleeding under DAPT, several studies have looked for alternatives to 12-month standard therapy. The GLOBAL LEADERS study, with a protocol of ticagrelor monotherapy after the first month after PCI and the TROPICAL-ACS study by de-escalation of prasugrel to clopidogrel, have attempted to reduce bleeding risk, but have not been able to demonstrate superiority compared to standard DAPT therapy.18,19 In the TWILIGHT study, a strategy of ticagrelor in monotherapy from three months after PCI demonstrated lower bleeding risk without an increase in ischemic events when compared to the classical strategy of 12 months of ASA plus ticagrelor.20
Furthermore, in DM patients the ischemic risk remarkably exceeds the hemorrhagic risk from the third month after the first two months after PCI, when the two risks seem to be balanced. There is still a lack of evidence regarding which antiplatelet has a better profile in DM and it is not an aim of this study to carry out a comparative analysis between ticagrelor and prasugrel in these patients. After the analysis of predictors of ischemic and hemorrhagic outcomes, a lower risk of bleeding was observed with prasugrel in our study. However, it is important to note that these results come from a univariate analysis and should only be interpreted as hypothesis generating. In a previous substudy of RENAMI, a significant reduction in bleeding events was observed with ticagrelor in DM population after propensity score matching analysis.21 Moreover, in the ISAR-REACT 5 trial, the incidence of death, myocardial infarction, or stroke was significantly lower among those who received prasugrel but no significant differences were found between both drugs in DM patients.22 More studies will be necessary to determine which antiplatelet is associated with better outcomes in this population.
Taking into account the excess ischemic risk in DM patients, new antithrombotic treatment strategies have been recommended by the newest European guidelines1,23 and prolonging DAPT beyond 12 months of standard therapy should be considered in high risk patients based on the results of DAPT and PEGASUS trials.24–26 Furthermore, the ATLAS-ACS-TIMI 51 and COMPASS trials have demonstrated benefit in adding an anticoagulant to conventional antiplatelet therapy in both ACS and stable atherosclerotic disease, achieving reduction in death and cardiovascular events at the cost of an increase in non-fatal bleeding.27,28 Additionally, antidiabetic drugs such as GLP-1 receptor agonists and SGLT2 inhibitors have also shown reduction of cardiovascular events and mortality and, therefore, they are highly recommended in DM patients.23 This is only the starting point for the investigation of new treatment regimens and targets to combat the unfortunate cardiovascular prognosis associated with the DM patient.
As a real clinical practice study, our results suggest that in the subgroup of DM patients without high hemorrhagic risk treated with ticagrelor or prasugrel, the main objective should be to achieve optimal platelet inhibition assuming a bleeding risk that does not appear to be significantly higher than that of the non-DM population.
LimitationsThis study is subject to several limitations. This is a retrospective observational study based on the clinical record, not a randomized trial, so our conclusions must be considered as hypothesis generating only. There is a lack of data regarding the kind of arterial access for PCI (radial or femoral) and therapeutic adherence, which may play a role in the incidence of both hemorrhagic and ischemic events. Some of the analyzes performed in this study may have been underpowered due to insufficient sample size and may have led to a type II statistical error explaining the lack of difference in bleeding rates between DM and non-DM patients. Moreover, it must be noted that when selecting only patients treated with prasugrel and ticagrelor, those patients with the highest bleeding risk – in whom the first choice antiplatelet would be clopidogrel – were excluded. Despite that fact, the low incidence of hemorrhagic events may reflect that there may exist some degree of underreporting. Therefore, our results may not be representative of all DM population. Despite careful multivariable adjustment and PS matching, selection bias is highly probable, and there may have been residual confounding.
ConclusionsThe ischemic-hemorrhagic balance of DM patients treated with DAPT with ticagrelor or prasugrel after ACS differs substantially compared to that of non-DM and depends on the time elapsed after PCI. The incidence of both hemorrhagic and ischemic events is higher in the first 30 days. Bleeding rates are similar in both groups, but reinfarction rates are higher in DM. While in non-DM patients the bleeding risk exceeds the risk of reinfarction between the third and the eleventh month after ACS, in DM patients the risk of reinfarction exceeds the bleeding risk throughout the first year, except for the first two months in which both risks are balanced. Our results suggest that in the subgroup of DM patients without high hemorrhagic risk, achieving optimal platelet inhibition should be the main objective, assuming a bleeding risk that does not appear to be significantly higher than that of the non-DM population.
- -
Bleeding and ischemic risk after acute coronary syndrome (ACS) is not uniform over time.
- -
DM has been associated with a worse prognosis, with a marked increase in reinfarctions and mortality.
- -
DM also increases the risk of bleeding, as a result of microangiopathy and endothelial damage secondary to hyperglycemia.
- -
To date, no study had balanced post-ACS ischemic-hemorrhagic risk in DM and non-DM patients while receiving dual antiplatelet therapy.
- -
In DM patients the risk of reinfarction exceeds the bleeding risk throughout the first year, except for the first two months in which both risks are balanced.
- -
In non-DM patients the bleeding risk exceeds the risk of reinfarction between the third and the eleventh month after ACS
The authors declare that this study has not received any funding.
Authors’ contributionAll authors declare to have made substantial contributions in each of the following aspects: the conception and design of the study, or the acquisition of data, or the analysis and interpretation of the data; the drafting of the article or the critical review of the intellectual content; the final approval of the version that is presented.
Conflicts of interestThe authors declare no conflicts of interest.
Abbreviations: ACS: acute coronary syndrome; ADIR: average daily ischemic risk; ADBR: average daily bleeding risk; DAPT: dual antiplatelet therapy; DM: diabetes mellitus; PCI: percutaneous coronary intervention.