Anatomically, the heart cannot be suspended in the thorax without structural continuity of the heart muscle to pump blood at a speed of 200cm/s with an efficacy that allows ejecting 70% of the left ventricular volume with only 12% shortening of its contractile unit, the sarcomere. The aim of this study was to investigate a) whether the myocardium is a single, continuous, helical muscle; b) the origin and end of the myocardial muscle; and c) the sliding movement between the myocardial segments during ventricular torsion–detorsion. This forces us to think of an antifriction mechanism.
MethodsA total of 18 hearts (10 bovine and 8 human) were used for anatomical and histological studies. Histological an histochemical analysis of the samples were performed, using the Alcian Blue technique to confirm hyaluronic acid and its antifriction mechanism.
ResultsThe myocardium could be defined as a single, continuous, and helical muscle that is inserted at its origin and end, according to the specimens analyzed, to an osseus-chondroid-tendinous nucleus called cardiac fulcrum, thus limiting the 2 ventricular chambers. Hyaluronic acid was found in the excision planes between the myocardial bundles.
ConclusionsThe myocardium in its longitudinal continuity adopts a spiraling spatial configuration, inserted at its end to a nucleus called cardiac fulcrum. The Thebesian veins and hyaluronic acid could have an essential role in the antifriction mechanism, due to the resistance between the sliding myocardial muscle surfaces in their torsion (systole) and detorsion (suction) motions.
Anatómicamente, el corazón no puede quedar suspendido en el tórax sin continuidad estructural del músculo cardiaco para bombear sangre a una velocidad de 200cm/s con una eficacia que permite expulsar el 70% del volumen del ventrículo izquierdo con solo un 12% de acortamiento de su unidad contráctil, el sarcómero. El objetivo de este estudio fue investigar: a) si el miocardio es un solo músculo helicoidal continuo; b) el origen y el final del músculo miocárdico, y c) el movimiento de deslizamiento entre los segmentos miocárdicos durante la torsión-destorsión ventricular. Esto fuerza a pensar en un mecanismo antifricción.
MétodosSe utilizaron un total de 18 corazones (10 bovinos y 8 humanos) para los estudios anatómicos e histológicos y todas las muestras se sometieron a análisis histológico e histoquímico. La tinción con alcian blue, un marcador fiable para identificar la presencia de ácido hialurónico, se utilizó para confirmar el mecanismo antifricción.
ResultadosEl miocardio se podría definir como un músculo único, continuo y helicoidal que se inserta en su origen y final a un núcleo óseo-condroide-tendinoso según las muestras analizadas, denominado fulcro cardiaco, limitando así las dos cámaras ventriculares. Se encontró ácido hialurónico en los planos de escisión entre los haces miocárdicos.
ConclusionesEl miocardio en su continuidad longitudinal adopta una configuración espacial en espiral, insertado en su extremo en un núcleo denominado fulcro cardiaco. Las venas tebesias y el ácido hialurónico podrían tener un papel esencial en el mecanismo antifricción, debido a la resistencia entre las superficies deslizantes del músculo miocárdico en sus movimientos de torsión (sístole) y detorsión (succión).
The function of the heart has a mechanical capacity that must be analyzed in terms of its structure. The spatial anatomical arrangement pattern of the myocardium is related to electrical propagation and torsion–detorsion motions.1 This analysis has led us to study its organic-functional integrity. This assumption was based on the hypothesis that to fulfill its function, the cardiac muscle must have structural continuity and a supporting point to apply its power. Anatomically, the heart cannot be suspended in the thorax without structural cardiac muscle continuity to pump blood at a speed of 200cm/s with an efficacy that allows ejecting 70% of the left ventricular volume with only 12% shortening of its contractile unit, the sarcomere.
Continuing with classical descriptions, we see that the anatomical attention of the heart was focused on external and internal surfaces, granting scarce importance to its intimate muscle structure. This was assumed as being solid and homogeneous, with a global uniform contraction, without taking into account that its mechanical capacity demanded a reinterpretation of its spatial anatomy and motion, leading to other topics of its functioning which were practically disregarded by cardiology.
Andreas Vesalius in his work “De Humani Corporis Fabrica” (1543) referred to the difficulty in identifying the myocardial layers. He expressed verbatim: “No matter how you perform the dissection of the meat of the heart, whether raw or cooked…, you can scarcely remove a portion of only one type of fiber, because they have multiple and different directions, especially transversal”.2
Three centuries later, this situation was also remarked by Pettigrew (1864) “Of the complexity of the arrangement I don’t need to say more than Vesalius, Haller and De Blainville; they all confessed their inability to decipher it”.3 Shaner in 1923 states that “the myocardium is formed by 2 flattened muscles with the shape of an 8. These muscles twist in opposite directions in systole, emptying their content”.4
The traditional anatomy of the heart considered that the muscle structure that made up the myocardium was homogeneous and compact composed of spiral muscles without a functional explication according to its real conformation. Recent interpretations pose controversial opinions, mainly between band and network models. Torrent-Guasp et al.5,6 in 1973 considered the myocardium as a cardiac muscle band, showing in numerous dissections that it is formed by a set of muscle fibers coiled unto themselves like a rope, flattened laterally, which by giving 2 spiraling turns define a helix limiting the 2 ventricles. MacIver et al.7 assumed that the ventricular walls are shaped by an intricate cardiomyocyte 3-dimensional network, implying that cardiomyocytes are arranged with radial and longitudinal angulations.
The aim of this work was to demonstrate through macroscopic dissection and histological studies that the myocardium is a single muscle with a helical anisotropic continuum alignment of the myocardial fibers, inserted at its origin and end to an osseus-chondroid-tendinous nucleus. All these anatomic-functional considerations may help both to quantify the severity of morbid processes as in therapeutic strategies.8
Methods- -
Cardiac dissection of 10 young (2-year-old) bovine hearts (800–1000g).
- -
Cardiac dissection of 8 human hearts: one from a 23-week gestation embryo; one from a 10-year-old patient (250g) and 6 adult hearts (mean weight: 300g).
- -
Histological and histochemical analysis of anatomical samples. Alcian blue technique was used in order to demonstrate the presence of hyaluronic acid, which has an antifriction mechanism with semiquantitative assessment.
This investigation, with human hearts from the morgue and with bovine hearts removed from the slaughterhouse, had the approval of the Ethics Committee of the institutions, involved in the work. Since human hearts are isolated pieces of the morgue, the Ethics Committee exempted them from informed consent. The investigations were conducted in accordance with the UK Animal Scientific Procedures Act 1996, the EU Directive 2010/63/EU for experimental animals and the “National Institutes of Health” guide for the care and use of laboratory animals (NIH Publication No. 8023, updated in 1978).
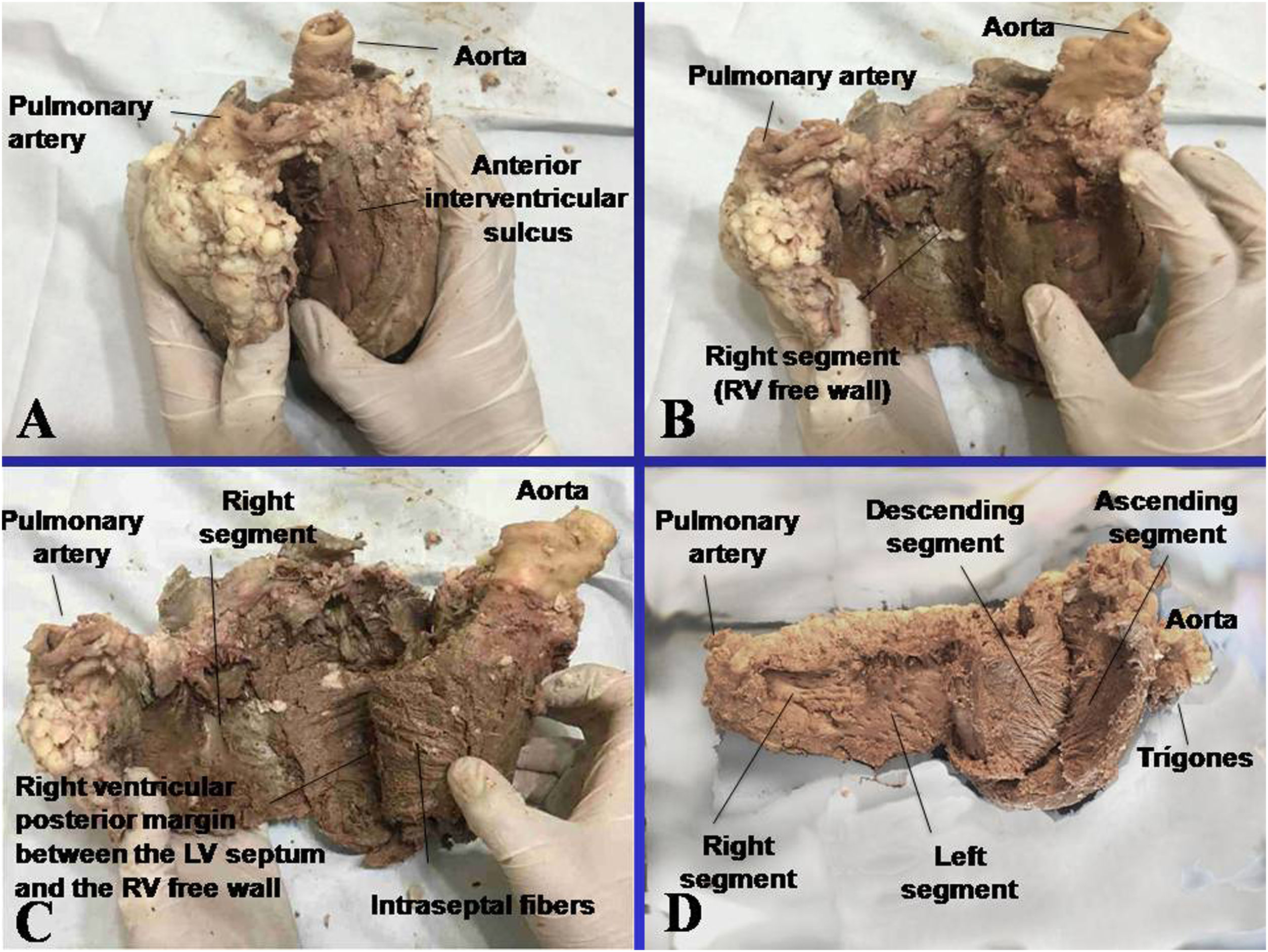
To dissect it, the heart must be boiled in water during 2h with the convenient addition of acetic acid (15mL per liter). This step removes the fat attached to the myocardium, making the dissection easier and neater. The aorta and the pulmonary artery are then cut at 3cm from their origin, separating the attachment between them, followed by a longitudinal incision at the level of the interventricular sulcus, on the superficial fibers extending transversally along the anterior wall of the ventricles. As there is only connective tissue between the atria and the ventricles, the denaturation process produced by heat allows the easy separation of these chambers.
The key maneuver to unfold the myocardium consists in entering the anterior interventricular sulcus with a blunt instrument, leaving on the left side of the operator the end of the myocardium corresponding to the pulmonary artery and the right segment. Next, traction is applied towards the same left side, completely releasing the pulmonary artery from the rest of the myocardium. This myocardial dissection reveals the cardiac fulcrum below and in front of the aorta, in a separate location from the right trigone and in an inferior plane to the origin of the right coronary artery, without continuity with the aortic valve and inserted as a complementary element between the aorta and the myocardium. This structure, point of attachment of the origin and end of the cardiac muscle, constitutes the insertion of the myocardium in a manner analogous to a skeletal muscle.
The next step (the most delicate one) consists in entering the dihedral angle between the right ventricular and intraseptal fibers. This separation from the right ventricle allows entering a cleavage between the anterior septal band and the intraseptal band (final segment of the continuous helical muscle), at the ventral part of the septum. Then, the dorsal part of the septum is dissected between the posterior septal band and the left descending segment to remove and separate the aorta.
Finally, the descending segment is blunty separated from that of the ascending segment, which leads to the cardiac fulcrum, at the right of the operator, allowing it to be extended in its full length. Fig. 1 shows the sequence of myocardial unfolding until it becomes a single, longitudinal muscle. Being able to unfold the myocardium with a similar thickness in all its length proves that it is a single and continuous muscle and not a heuristic construction.
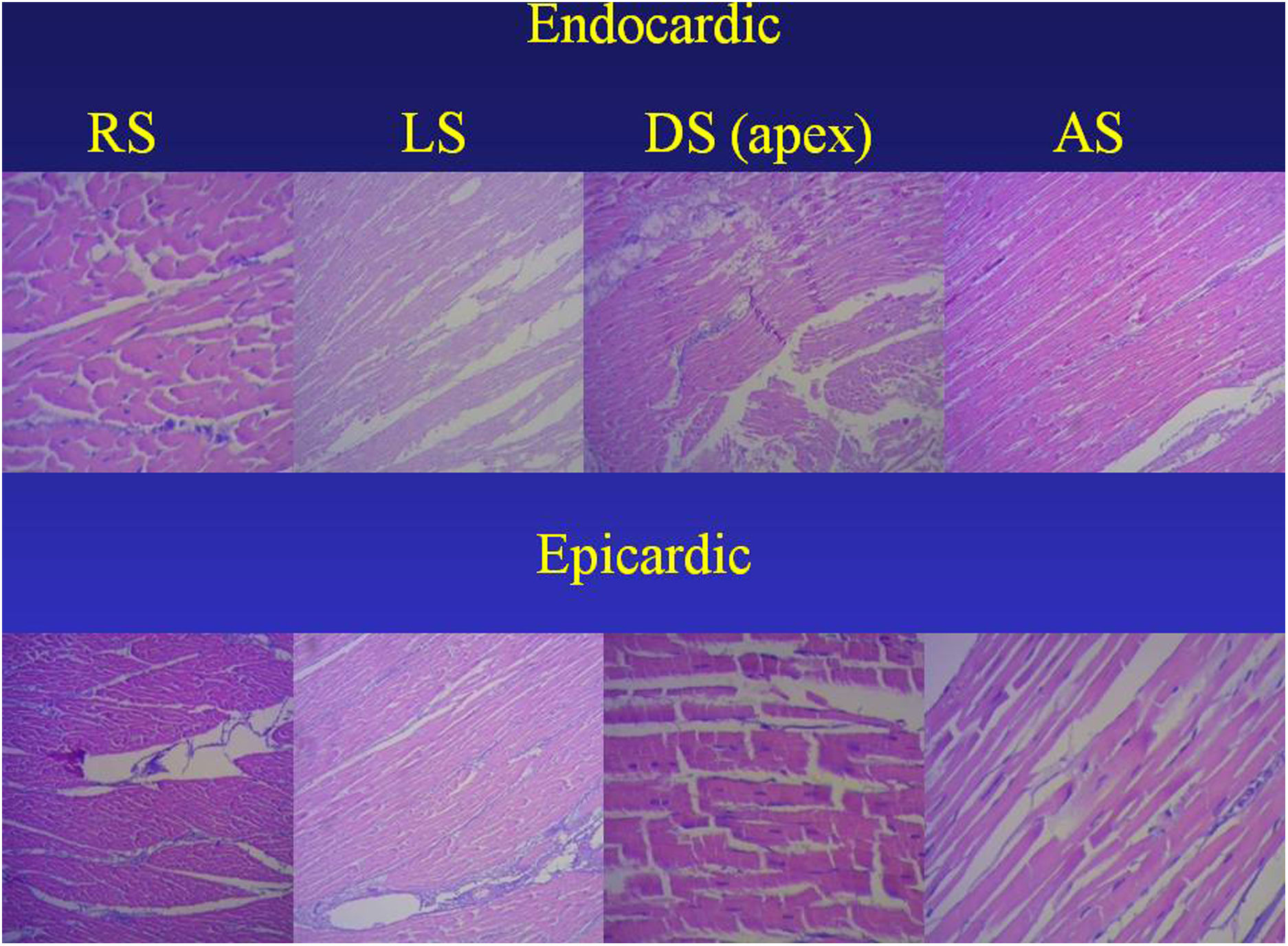
ResultsAnatomical and sequential histological analysis of the myocardiumThe sequential histological analysis of the unfolded myocardium shows the longitudinal orientation of fibers in agreement with the continuity of segments resulting from its spatial arrangement, an orientation hat is parallel. This situation is evident in the internal and external surfaces of each segment (Fig. 2).
Sequence of segments of histological analysis of myocardium (bovine heart). The orientation of the endocardial and epicardial surfaces of each segment is evident in the uncoiled myocardial helix. Hematoxylin–eosin stain (15×). AS, ascending segment; DS, descending segment; LS, left segment; RS, right segment.
In our studies, no segment of the histological sequence corresponding to the longitudinal continuity of the myocardium presents a network configuration. In the external surface of the distal end of the descending segment, when it turns at the apex and becomes the ascending segment, the orientation of the cardiomyocytes in longitudinal sections generates a dissimilar architecture to that of the internal surface, only place where this situation occurs. This arrangement is found at the apex, where the spiral course of the myocardial fibers that shift from the periphery towards the center determines a sudden change in direction, with subepicardial fibers becoming subendocardial and overlapping like the tiles of a roof, as evidenced in Fig. 2. This configuration is logical for the demonstration of the helical arrangement, since it occurs at the site where the myocardium abruptly changes its direction from descending to ascending, being parallel in the rest of its organization. This resembles the Moebius band due to the progressive change in fiber angulations, turning them from epicardial to endocardial.
The myocardial structure is not a network but a continuous muscle with a helical anisotropic disposition of the myocardial fibers. The network concept originated from the myocardial helical coil resulting in segment overlap, which function independently with friction between their surfaces due to their opposite movements (Fig. 3C). This sliding movement implies finding a lubricating system consisting of hyaluronic acid which would facilitate the independent movement of overlapping segments with lower energy expenditure.9–11 This arrangement is essential to achieve myocardial torsion–detorsion, the fundamental action of cardiac mechanics that would be impossible with a network structure (crisscross of myocardial fibers). The Thebesian system is formed by venous courses and lacunar spaces, bathed in hyaluronic acid, allowing it to cross the myocardial width formed by the coiled cardiac muscle with attachment of the different segments in its continuity (Fig. 4C).
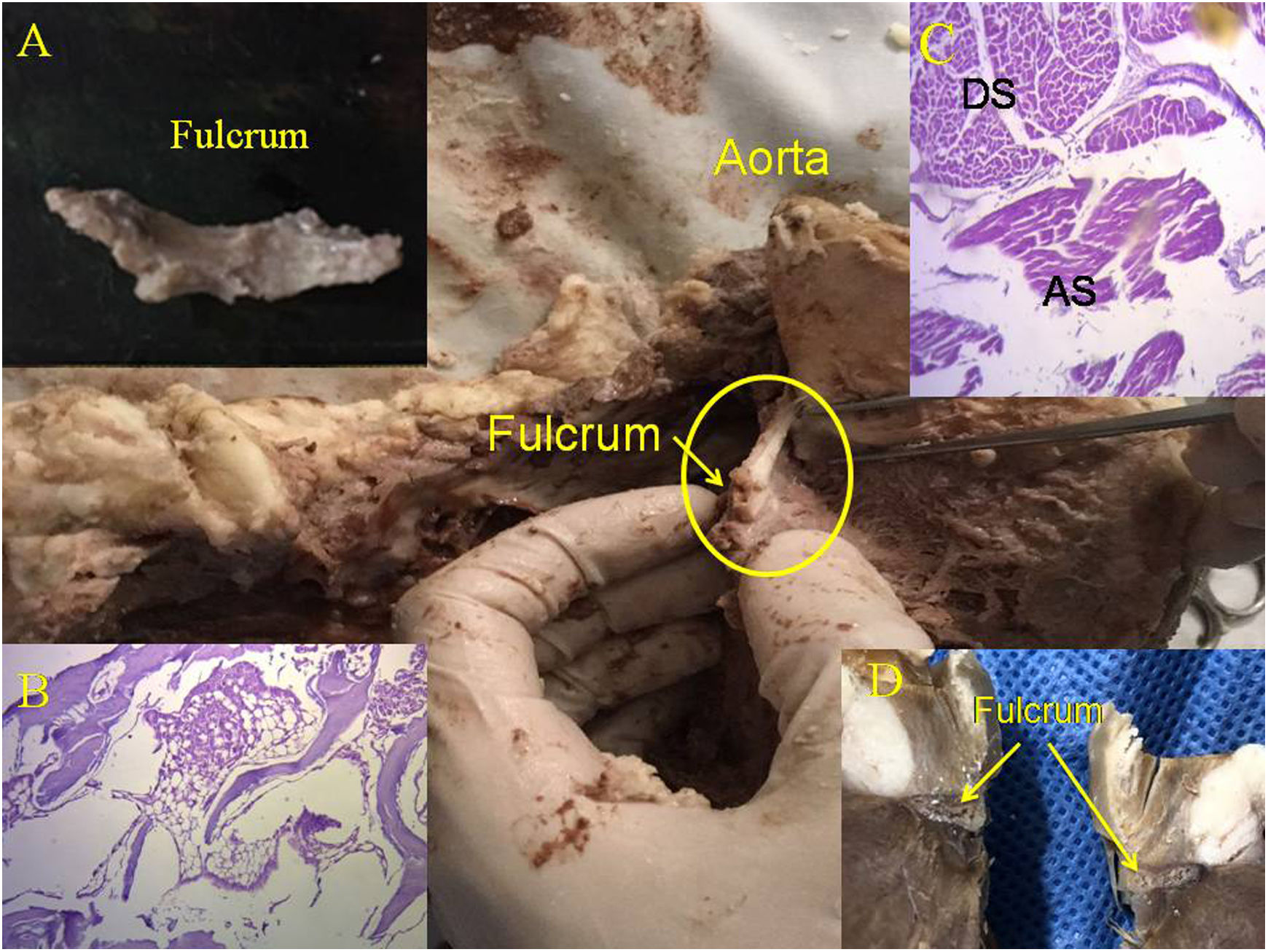
Cardiac fulcrum (bovine heart). (A) Resected piece of the cardiac fulcrum; (B) mature trabecular bone forming the fulcrum. Hematoxylin–eosina stain at low magnification (10×); (C) the histology shows the different orientation of the longitudinal fibers of the AS in relation to the transverse fibers of the descending segment; (D) cardiac fulcrum in other view. AS: ascending segments; DS: descending segments.
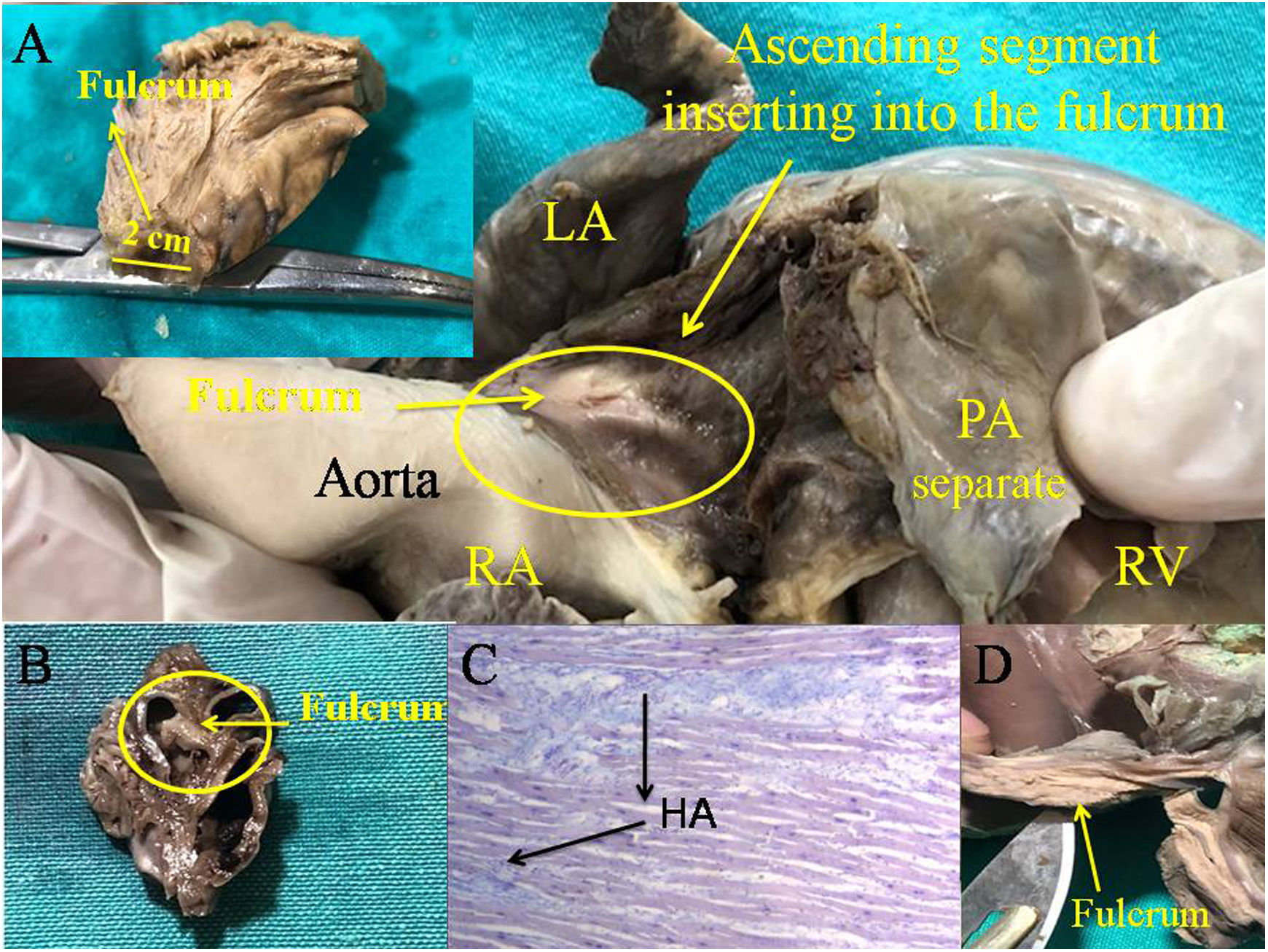
Cardiac fulcrum (human heart). (A) 10-year-old; (B) 23 week gestation human embryo heart; (C) interstitial space between cardiomyocytes showing HA, technique with Alcian blue (15×) (human adult heart), (D) cardiac fulcrum resected (adult human). HA: hyaluronic acid; LA: left atrium; PA: pulmonary artery; RA: right atrium; RV: right ventricle.
At the beginning of myocardial muscle unfolding, the first interruption corresponds to a chondroid nucleus where both ends of the myocardium meet and which we have termed cardiac fulcrum.12 After this point, the ends of the myocardium separate. The right segment (origin of the myocardium) attaches mainly to the anterior surface of the fulcrum. The ascending segment, (end of the myocardium), enters the fulcrum mainly through its inferior portion (Fig. 3 and Fig. 4). In this maneuver the myocardium loses its integrity, and its fundamental structure disappears.
Histological analysis of the cardiac fulcrum and trigonesWe have found in all bovine and human heart anatomical investigations a nucleus whose histological structure is different according to the specimens analyzed, presenting itself with an osseous, chondroid or tendinous consistency. In this structure, both the muscle fibers of the ascending segment (final insertion) and those of the right segment (initial insertion) are attached.
This attachment, which we have called the fulcrum, is located in the vicinity of the tricuspid valve (right), the aorta (posterior) and the pulmo-tricuspid cord (anterior). To find it, it is necessary to bring the pulmonary artery, the pulmo-tricuspid cord and the right segment to the observer's left side, stripping the root of the aorta. This reveals the fulcrum in front of the aorta and inferior to the right trigone and to the origin of the right coronary artery, detached from the aortic continuity and located as a complementary element between the aorta and myocardium.
The existence of the “os cordis”, an osseus formation found in bovine and sheep hearts is a fact mentioned in veterinary medicine. It is located in the same place where we have investigated this structure, both in bovines and humans. Beyond its mere mention in bovines, it was never assigned any function, or the sense of its presence understood, and it also lacks description in humans.
The consistency of the cardiac fulcrum in bovine hearts, osseus on palpation, has been observed by histology (Fig. 3). Its size, corroborated by dissection and computed tomography, is approximately 37 to 45mm×15mm and triangular in shape. The microscopic observation of the bovine cardiac fulcrum shows a trabecular osteochondral matrix. Its general structure resembles the metaphyseal growth of the long bones and increased magnification reveals bone trabeculae with osteoblasts and segmental lines secondary to bone apposition. The same histological results have been found in chimpanzees.13
The histological findings of the cardiac fulcrum in the human heart correspond to the early age of 10 years. In this sample, the fulcrum shows a central zone formed by chondroid tissue. Given the age of the heart, it is logical that the fulcrum is smaller and characterized by more chondroid than osseous tissue. This finding was repeated in the 23-week-old human fetus with the characteristic prechondroid bluish areas in a myxoid stroma (Fig. 4 and Fig. 5).
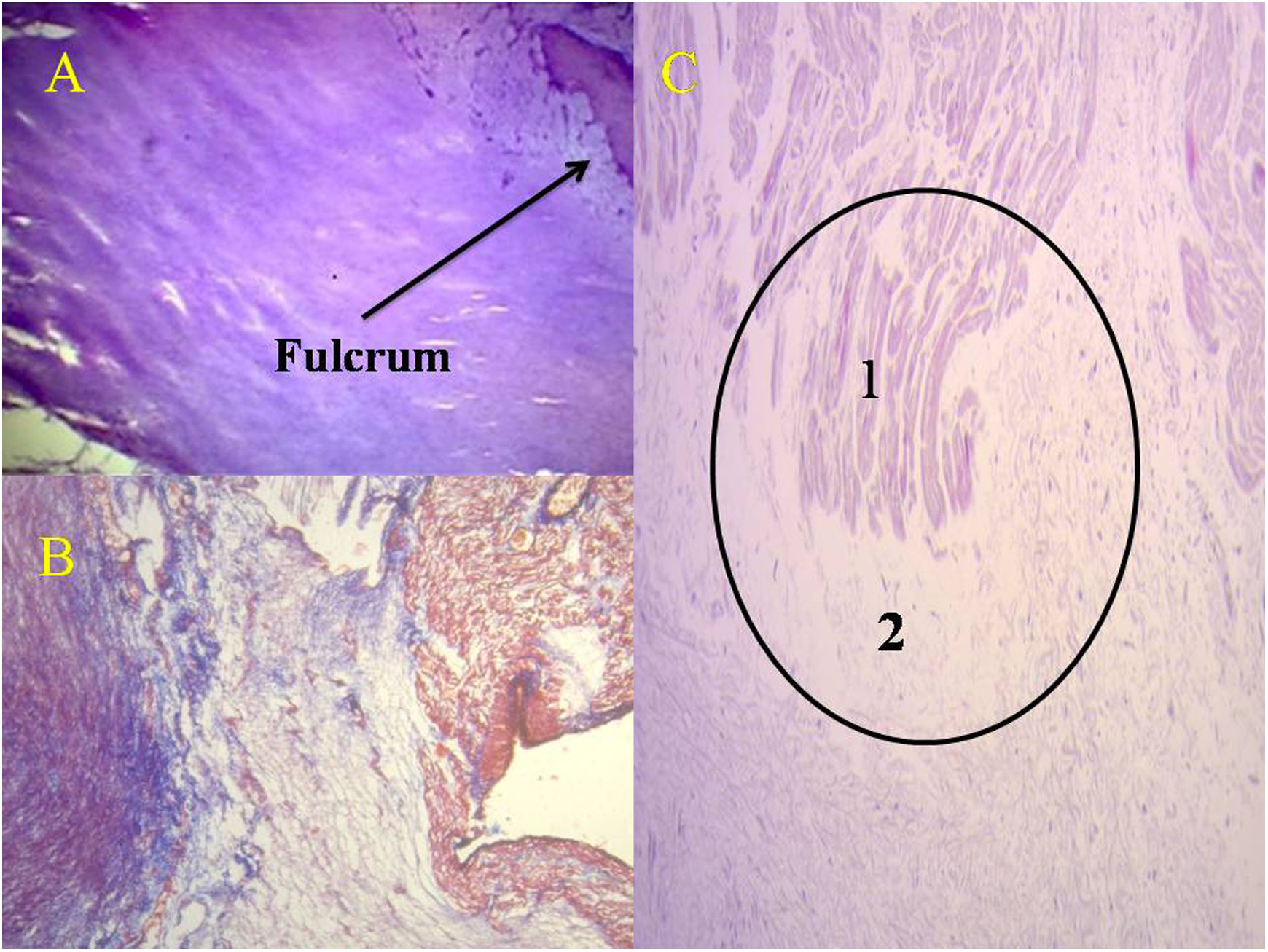
(A) Ten year old human heart. Cardiac fulcrum formed by chondroid tissue. Hematoxylin–eosin stain (15×). (B) Fulcrum in a 23-week gestation fetus. It is noted prechondroid bluish areas in a myxoid stroma. Masson's trichrome staining technique (15×); (C) cardiomyocytes penetrating in the cardiac fulcrum (adult human heart). The circle details the insertion site. 1: cardiomyocytes; 2: fibrocolagenus matrix. Hematoxylin–eosin stain (15×).
However, in the histological analysis of the adult human heart fulcrum (measuring approximately 25mm×15mm), a tendinous collagen matrix was found, which needs further explanation (Fig. 4). We have found in all investigations that the site of the cardiac fulcrum has been similar in location and morphology.
They present insertion of the myocardium into the rigid structure of the fulcrum, integrating a cardiomyocytic-matrix unit, regardless of their osseus, cartilaginous or tendinous nature in the different specimens studied (Fig. 5C). This fixation point implies that, as in any muscle, it acts as a lever and also as a bearing, preventing the ventricular rotational force, either by torque or torsion, from spreading to the great vessels, thus dissipating the energy produced by the helical motion of the muscle.
To faithfully establish the identity of the cardiac fulcrum, histological analysis has also been carried out on the trigones, trying to find cardiomyocytes as a possible insertion of the cardiac muscle in these structures. In our investigation, only collagenous tissue without cardiomyocytes was observed in the trigones, confirming that the fulcrum is the support of the myocardium, both at its origin and at its end.
DiscussionThis study provides evidence that myocardial fibers constitute a continuous muscle that describes a double helix to form both ventricles and that, to fulfill its function, the muscle needs a supporting point that we have found and named the cardiac fulcrum. The myocardium has the following characteristics derived from the anatomical–histological–functional analysis performed:
- -
It is made up of a single, continuous, and coiled muscle that forms a helix with 2 turns.
- -
It is attached at its origin and end, just as any muscle does, to a support that we have described and called the cardiac fulcrum.
- -
The anisotropic spatial helical arrangement forces the muscle to overlap segments in its configuration.
- -
This anatomical situation has a deep correspondence with the motions and the stimuli that run through its segments.1
- -
Cardiac function is related to this functional anatomical arrangement.
- -
The transverse interconnections between the tracts do not rule out the concept of continuous myocardium, understanding this arrangement as the result of the evolutionary development to obtain a solid structure.
Therefore, the myocardium can be defined as a single muscle that in its longitudinal continuity adopts a spiraling spatial configuration, inserted at its ends (origin and end) in an osteochondral-tendinous nucleus according to the specimens analyzed, called the cardiac fulcrum. In this way it defines the 2 ventricular cavities.
The myocardium as a single, helical muscle is not represented by the word “band”. Torrent-Guasp's concept of band does not correspond to the etymology of the word and to a complete spiraling individuality of its course where it is obliged to overlap its segments. Perhaps the selection of this word has been unfortunate. Band is an elongated strip that goes from one end to the other and is identified on the surface on which it appears, with well-defined limits and does not assume the integrity of a structure. It is a part of it, a situation that does not occur in the myocardium, since the muscle that constitutes it is a whole.
Neither can it be considered a network since this structure is not related to the functional anatomy of the heart. There are criteria that support the concept of continuity of the myocardium as a single, continuous and spiraling muscle:
- -
Muscle homogenization hides the real continuity by overlapping its segments. This implies considering that its structural solidity is required in birds and mammals to ensure that the blood is ejected at a high speed in a limited time, through an organ that must supply 2 circulations (systemic and pulmonary). The anatomical investigation of the heart through adequate dissection, histological exploration, imaging procedures obtained by echocardiographic analysis, and electrophysiological studies carried out with three-dimensional electroanatomical mapping and with diffusion-tensor magnetic resonance imaging shows the continuous muscular path that defines the 2 ventricles.14–18
The unfolded myocardium shows to have the same thickness throughout its extension. When it is folded, it can be seen that the thickness of the right ventricle is less than that of the left ventricle, since the former is made up of only one segment of the muscle, while the latter presents the attachment of 2 segments.
- -
Its dissection always reaches the same point of unfolding. The question is: how can you always repeat dissections with the same result and say that it is a fallacy?
- -
Its function leads it to have a supporting point as any skeletal muscle, both at its origin and at its end. If the myocardium did not have this helical spatial anatomical conformation, was not attached at both its ends at the base of the heart and did not remain free at the apex, that is to say, as a pendulum in the thorax, and if it did not present a stimulation that allows its torsion and detorsion motions, it would not be able to fulfill its extraordinary muscular power (video of the supplementary data).19–22 Ultrasound with speckle-tracking techniques has demonstrated the sliding of one segment over another and the movements of shortening and lengthening during the systolic and suction phases, respectively.23Torsion in a healthy heart ranges between 15 and 20° according to age.24
Richard Lower in 1669, mentioned by Henson et al., 25 considered that the myocardium was subjected to a torsion motion related to the helical fibers that formed it. He expressed that the heart exerted a motion similar to “wringing a towel” and not as it was considered since Harvey who claimed it was due to ventricular radial compression with the analogy of “closing a fist”. This concept was later studied and tested in humans and mice by Henson et al.25 The heart achieves the ejection of its content by torsion of its walls and initiates its filling through detorsion. Torsion together with ventricular longitudinal shortening can be explained through the helical arrangement and continuity of the cardiac muscle.
- -
Cardiac function cannot be explained by a network configuration.26 In this regard, Maclver's work states: “None of the histological studies of the myocardium that we are aware, in contrast have provided any evidence for an origin and insertion as described for the alleged unique myocardial band” and “None of these investigations have provided any evidence of an alignment of the cardiomyocytes that follows the course of the unique myocardial band.”7 First of all, our published results describing the presence of a cardiac fulcrum in human and animal hearts12 describe the cardiac support that, in the words of Maclver, would give rise to that single, continuous, helical muscular conformation of the heart. Regarding the second conclusion of this author, we have investigated and analyzed in the results of our work the sequential anatomical-histological examination of the heart muscle (Fig. 2).
- -
The trigones do not show cardiomyocyte insertion, confirming that the only attachment of the myocardium is the fulcrum.
In the face of this controversy, imaging procedures have been used, getting closer to its resolution, but by not acting directly on the anatomy and histology, they have not been able to define essential descriptions of its conformation and function. We have used fresh bovine and human hearts to obtain detailed descriptions to elucidate the true spatial myocardial architecture.
LimitationsThe human specimens studied were scarce because it is difficult to obtain intact, well-preserved hearts for meticulous dissection. We consider that the work should be expanded with a greater number of adult and especially child hearts. Our research was limited to 18 specimens, 8 human and 10 bovine hearts.
ConclusionsThe myocardium is a single muscle that assumes an anisotropic spiraling spatial configuration in its longitudinal continuity.
It is attached at its extremities (origin and end) to an osteochondral-tendinous nucleus, according to the analyzed specimens, called cardiac fulcrum.
The opposing sliding motion of the left ventricular internal segments in relation to the external segments to achieve the mechanism of ventricular torsion, generates inevitable friction between them. From the point of view of physics, this friction implies an opposition to motion. This would entail a high energy cost if the heart did not have a spongy system, with the participation of Thebesian and Langer venous ducts, and a lubricating antifriction system. The histological studies of this spongy matrix and its ducts revealed that the antifriction effect is hyaluronic acid flowing across the myocardial thickness.
FundingNo funding.
Authors’ contributionsJ. Trainini: director of research. M. Beraudo: anatomical dissection. M. Wernicke: pathology. F. Carreras Costa: cardiac magnetic resonance. A. Trainini: anatomical disecction. V. Mora Llabata: echocardiography. J. Valle Cabezas: hydraulics. D. Lowenstein Haber: tomography. M.E. Bastarrica: clinic coordination. J. Lowenstein: echocardiography.
Conflicts of interestThe authors declare that they have no conflict of interest.
Torrent-Guasp considered the myocardium as a cardiac muscle band, showing in numerous dissections that it is formed by a set of muscle fibers coiled unto themselves similar to a rope, flattened laterally, which by giving 2 spiraling turns define a helix limiting the 2 ventricles.
But nevertheless, anatomically, the heart cannot lie suspended in the thorax without structural cardiac muscle continuity to pump blood at a speed of 200cm/s with an efficacy that allows ejecting 70% of the left ventricular volume with only 12% shortening of its contractile unit, the sarcomere.
Does it contribute anything new?The myocardium is a single muscle that assumes an anisotropic spiraling spatial configuration in its longitudinal continuity.
It is attached at its extremities (origin and end) to an osteochondral-tendinous nucleus, according to the analyzed specimens, called cardiac fulcrum.
The opposite movement of the ascending and descending segments, and also of the latter against the septal region of the myocardium, would generate friction between their sliding surfaces in their torsion (systole) and detorsion (suction) motion. Therefore, the Thebesian veins and hyaluronic acid could have a major role as an antifriction mechanism.