In patients with previous myocardial infarction (MI) guidelines recommend a low-density lipoprotein cholesterol (LDL-C) < 70mg/dL (ACC/AHA) or < 55mg/dL (European Society of Cardiology). Current data on individual variation of LDL-C in this population is limited. We aimed to assess the percentage of patients who achieved an LDL-C below target, the variation from 2013 to 2018 and to evaluate clinical predictors of good LDL-C control.
MethodsA retrospective cohort study was conducted. All Cardiology outpatients with previous MI with available LDL-C measurements after MI in 2013, 2016 and 2018 were included. We estimated the prevalence of patients with LDL-C below target for each year, individual trends and performed multivariate analysis for assessing clinical predictors.
ResultsOf 650 patients, an LDL-C < 70mg/dL was attained in 24.8% and 34.0% in 2013 and 2018, respectively, with a median reduction in LDL-C of 6mg/dL (P<.001). LDL-C < 55mg/dL was observed in 8.0% and 11.7% in the same years. Only 10.0% and 1.4% of patients presented 3 LDL-C measurements < 70mg/dL and 55mg/dL, respectively, whereas 78.8% and 47.4% did not have any LDL-C < 55mg/dL or < 70mg/dL. Male sex and diabetes were constant predictors of achieving an LDL-C below target.
ConclusionsThere has been an improvement in LDL-C control in recent years, but only one-third of the outpatients with previous MI are currently on LDL-C < 70mg/dL and even less on LDL-C < 55mg/dL. Male sex and diabetes predicted better LDL-C control.
En pacientes con infarto de miocardio (IM) previo, se recomienda un colesterol unido a lipoproteínas de baja densidad (cLDL) <70mg/dl (ACC/AHA) o <55mg/dl (European Society of Cardiology). Los datos actuales sobre la variación individual de cLDL en esta población son limitados. Nuestro objetivo fue evaluar el porcentaje de pacientes que alcanzaron el objetivo de cLDL, la variación de 2013 a 2018 y evaluar predictores clínicos para el buen control de cLDL.
MétodosSe realizó un estudio de cohorte retrospectivo que incluyó a los pacientes ambulatorios de cardiología con IM previo con mediciones de cLDL disponibles después del IM en 2013, 2016 y 2018. Se estimó la prevalencia de pacientes con cLDL por debajo del objetivo para cada año, las tendencias individuales y realizamos un análisis multivariado para evaluar predictores clínicos.
ResultadosDe 650 pacientes, el 24,8 y 34,0% alcanzaron un cLDL <70mg/dl en 2013 y 2018, respectivamente, con una reducción media de 6mg/dl (p<0,001). Se observó un cLDL <55mg/dl en el 8,0 y 11,7% en los mismos años. El 10,0 y el 1,4% de los pacientes presentaron las 3 mediciones de cLDL <70mg/dl y <55mg/dl, respectivamente. El sexo masculino y la diabetes fueron predictores de lograr el objetivo de cLDL.
ConclusionesHa habido una mejora en el control de cLDL en los últimos años, pero un tercio de los pacientes ambulatorios con IM previo presentan actualmente un cLDL <70mg/dl, y un porcentaje menor aún un cLDL <55mg/dl. El sexo masculino y la diabetes predijeron un mejor control de cLDL.
Low-density lipoprotein cholesterol (LDL-C) levels have been strongly correlated with major adverse cardiovascular events (MACE) and all-cause mortality1–3 Since patients with coronary heart disease are at very high risk for MACE, current ACC/AHA and previous European Society of Cardiology (ESC) guidelines recommended an LDL-C < 70mg/dL in this target population.4,5 New ESC guidelines released in 2019 recommend an LDL < 55mg/dL.6
Statins are the cornerstone of secondary prevention, and many randomized clinical trials have shown a consistent LDL-C reduction associated with lower MACE.7–9 Drugs different from statins have been developed in recent years. IMPROVE-IT trial, published in 2016, showed the benefit of adding ezetimibe to statins for lowering LDL-C and MACE.10 FOURIER and ODYSSEY-OUTCOMES trials, published in 2017 and 2018, respectively, have also proved that evolocumab and alirocumab, 2 monoclonal antibodies against the proprotein convertase subtilisin/kexin 9 receptor, lead to even better outcomes in patients at very high risk who are already on high statin dose.11,12 The improvement of cardiovascular outcomes has been reported to be independent from the drug used to lower LDL-C levels.2
Several national and international registries conducted among the high-risk population, mainly transversal or short-term longitudinal follow-up studies, have repeatedly shown deficient attainment of guideline-specified goals.13–20 There is some evidence of improvement in LDL-C control until 201421,22 but data is limited regarding the impact of the recent randomized clinical trials on LDL-C control from 2014 until now.
We aimed to estimate the contemporary prevalence of patients with previous myocardial infarction (MI) who achieved an LDL-C < 70mg/dL during follow-up as recommended by previous ESC guidelines, the individual trends in LDL-C target attainment from 2013 to 2018, and to evaluate clinical predictors for proper LDL-C control, regardless of the treatment used. We also explored the prevalence of LDL-C < 55mg/dL in this very high-risk cohort.
MethodsStudy design and patient populationWe conducted a retrospective cohort study. All consecutive Cardiology outpatients visited from May 2014 to July 2016 in a primary healthcare network that gives support to a 250,000-population area were included. We selected LDL-C from 2013 as baseline, and LDL-C from 2016 and 2018, the period in which the aforementioned landmark randomized clinical trials were published. We included all patients who had a MI before the first LDL-C measurement in 2013 and who had all 3 LDL-C measurements available from clinical records. For patients who were dead at time of data collection no LDL-C could be obtained due to technical difficulties, so they were excluded from the analysis. No restrictions existed in this area during the study period for the prescription of high-intensity statin or ezetimibe, and proprotein convertase subtilisin/kexin 9 antagonists were available for those patients with LDL-C ≥ 100mg/dL under maximum tolerated LDL-C lowering treatment. Data collection was authorized for our local ethics committee (Hospital Universitari Vall d’Hebron).
Data collectionDemographic and clinical characteristics at the time of the index visit were collected retrospectively from clinical records, including age, sex, weight, height, hypertension, dyslipidemia, diabetes mellitus, peripheral artery disease, cerebrovascular disease, age at first MI, years after first MI, atrial fibrillation and left ventricle ejection fraction. LDL-C values according to Friedewald formula were obtained from blood tests performed in 2013, 2016 and 2018. If no LDL-C was available from the selected year, we selected a blood test from 6 months earlier or after, but always at least 3 months after the first MI to ensure proper secondary prevention measures were established.
Statistical analysisResults are presented as absolute and relative frequencies for categorical variables and as mean and standard deviation or, when appropriate according to Saphiro–Wilk test, as median and interquartile range for continuous variables. Depending of normality, Student t, U-Mann Whitney or Wilcoxon tests (when applying for matched variables such as LDL-C in 2013 and 2018) were performed when comparing continuous variables, and chi-square with Bonferroni-like adjustment was performed for the comparison of categorical variables. Multivariate analysis was performed with multiple linear regression for quantitative dependent variables and binary logistic regression for dichotomous variables. All dependent variables that showed a P<.1 in univariate comparisons in any of the 3 years assessed were included in the multivariate analysis. Statistical significance was established as a two-tail P<.05. All statistical analysis was performed with Stata 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
ResultsA total of 854 outpatients alive at time of data collection with previous MI before 2013 were found. However, 204 patients had no LDL-C available in some of the selected years and were excluded for the analysis. Finally, we could obtain all necessary data from 650 patients.
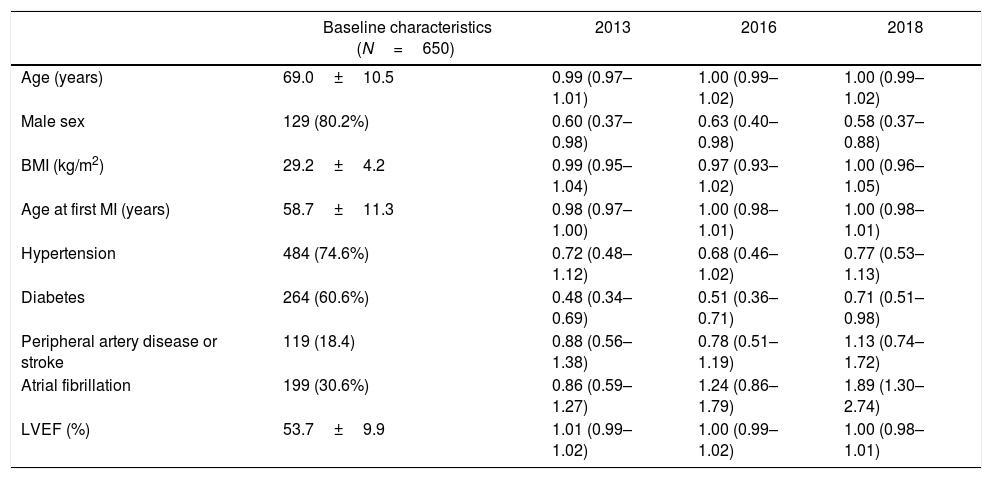
Baseline characteristics of our cohort at the time of the index visit and its capacity to predict, in a univariate test, an LDL < 70mg/dL in each year are shown in Table 1. Mean age was 69.0 years old and 80.2% were male. Of note, there was a high prevalence of comorbidities (40.2% had diabetes and 30.4% had atrial fibrillation) and first MI occurred a median of 7 years (interquartile range: 2–13 years) before 2013.
Baseline characteristics are shown on the left column as mean±standard deviation for continuous variables or, when categorical, as absolute frequencies with its relative frequencies. For every clinical variable, an odds ratio (95% confidence interval) for predicting an LDL-C < 70mg/dL in each year is provided in the univariate analysis.
| Baseline characteristics (N=650) | 2013 | 2016 | 2018 | |
|---|---|---|---|---|
| Age (years) | 69.0±10.5 | 0.99 (0.97–1.01) | 1.00 (0.99–1.02) | 1.00 (0.99–1.02) |
| Male sex | 129 (80.2%) | 0.60 (0.37–0.98) | 0.63 (0.40–0.98) | 0.58 (0.37–0.88) |
| BMI (kg/m2) | 29.2±4.2 | 0.99 (0.95–1.04) | 0.97 (0.93–1.02) | 1.00 (0.96–1.05) |
| Age at first MI (years) | 58.7±11.3 | 0.98 (0.97–1.00) | 1.00 (0.98–1.01) | 1.00 (0.98–1.01) |
| Hypertension | 484 (74.6%) | 0.72 (0.48–1.12) | 0.68 (0.46–1.02) | 0.77 (0.53–1.13) |
| Diabetes | 264 (60.6%) | 0.48 (0.34–0.69) | 0.51 (0.36–0.71) | 0.71 (0.51–0.98) |
| Peripheral artery disease or stroke | 119 (18.4) | 0.88 (0.56–1.38) | 0.78 (0.51–1.19) | 1.13 (0.74–1.72) |
| Atrial fibrillation | 199 (30.6%) | 0.86 (0.59–1.27) | 1.24 (0.86–1.79) | 1.89 (1.30–2.74) |
| LVEF (%) | 53.7±9.9 | 1.01 (0.99–1.02) | 1.00 (0.99–1.02) | 1.00 (0.98–1.01) |
LDL-C, low-density lipoprotein cholesterol; BMI, body mass index; MI, myocardial infarction; LVEF, left ventricle ejection fraction.
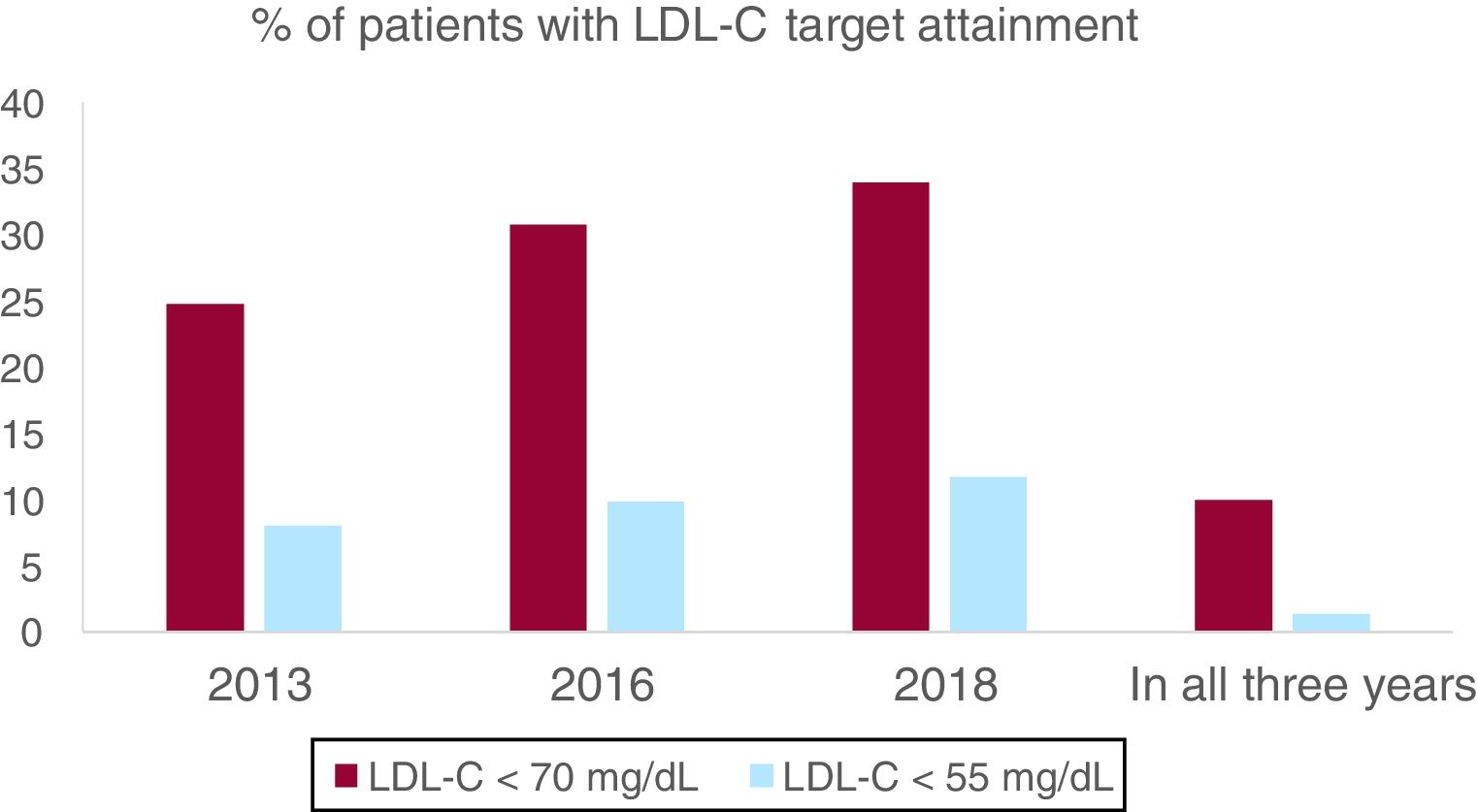
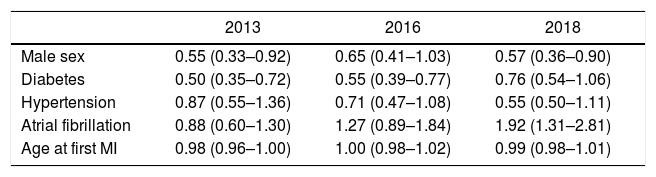
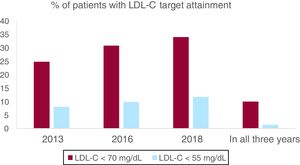
The percentage of patients with LDL-C < 70mg/dL was 24.8% in 2013, 30.8% in 2016 and 34.0% in 2018, with an absolute increase of 9.2% from 2013 to 2018 (P<.001). Patients with an LDL-C ≥ 100mg/dL decreased from 26.6% in 2013 to 20.5% in 2018 (P<.001). Median LDL-C was higher in 2013 than it was in 2018 (86 [70–101] mg/dL in 2013 vs 80 [66–96] mg/dL in 2018; P<.001). After adjustment in the multivariate analysis, only male sex, diabetes and no history of atrial fibrillation remained significant (Table 2). To ensure that patients with a MI in 2013 were not undertreated and acting as a confounding factor, we compared patients with a MI in 2013 (N=67, 10.3%) with patients with a MI in the previous years (N=583, 89.7%), and no difference could be seen in percentage of patients with LDL < 70mg/dL (24.2% vs 29.8% respectively, P=.31). Among all clinical variables analyzed, there were no predictors for LDL-C variation from 2013 to 2018, given that LDL-C uniformly lowered in all subgroups.
Odds ratio (95%CI) for the capacity of each clinical variable to predict an LDL-C < 70mg/dL in each year in a multivariate analysis.
| 2013 | 2016 | 2018 | |
|---|---|---|---|
| Male sex | 0.55 (0.33–0.92) | 0.65 (0.41–1.03) | 0.57 (0.36–0.90) |
| Diabetes | 0.50 (0.35–0.72) | 0.55 (0.39–0.77) | 0.76 (0.54–1.06) |
| Hypertension | 0.87 (0.55–1.36) | 0.71 (0.47–1.08) | 0.55 (0.50–1.11) |
| Atrial fibrillation | 0.88 (0.60–1.30) | 1.27 (0.89–1.84) | 1.92 (1.31–2.81) |
| Age at first MI | 0.98 (0.96–1.00) | 1.00 (0.98–1.02) | 0.99 (0.98–1.01) |
LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction.
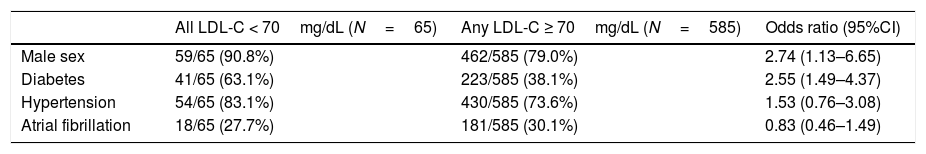
When we analyzed maintenance of LDL-C < 70mg/dL, only 10.0% of the patients included had an LDL-C < 70mg/dL in all 3 measurements assessed, and just 40.5% of the patients with an LDL-C < 70mg/dL in 2013 maintained their LDL-C below this target in successive measurements. Moreover, 47.4% of the patients had none of the assessed LDL-C < 70mg/dL. When we performed a logistic regression to evaluate what clinical factors could predict achieving an LDL-C < 70mg/dL in all measurements, only male sex, and diabetes remained significant predictors (Table 3). LDL-C < 70mg/dL in all assessments was achieved in only 5% of women and 11% of men (P<.01 for the difference between sex). 56% of woman and 45% of men had all LDL-C assessed ≥ 70mg/dL (P<.01 for the difference between sex).
On the left, absolute and relative frequencies of each clinical predictor are shown for patients with all 3 assessed LDL-C < 70mg/dL (N=65), and for patients with any LDL-C ≥ 70mg/dL. On the right column, odds ratio (95%CI) to predict all LDL-C < 70mg/dL is provided for each clinical variable in a multivariate analysis.
| All LDL-C < 70mg/dL (N=65) | Any LDL-C ≥ 70mg/dL (N=585) | Odds ratio (95%CI) | |
|---|---|---|---|
| Male sex | 59/65 (90.8%) | 462/585 (79.0%) | 2.74 (1.13–6.65) |
| Diabetes | 41/65 (63.1%) | 223/585 (38.1%) | 2.55 (1.49–4.37) |
| Hypertension | 54/65 (83.1%) | 430/585 (73.6%) | 1.53 (0.76–3.08) |
| Atrial fibrillation | 18/65 (27.7%) | 181/585 (30.1%) | 0.83 (0.46–1.49) |
CI, confidence interval; LDL-C, low-density lipoprotein cholesterol.
LDL-C < 55mg/dL was attained only in 8% patients in 2013 and in 11.7% patients in 2018 (P<.001), but only 1.4% (9 patients, all male) maintained their LDL < 55mg/dL in all 3 measurements (Fig. 1). 78.8% of the patients had none of the LDL-C measurements < 55mg/dL. Male sex and diabetes remained clinical predictors both for attaining LDL-C target in a single year and for maintaining an LDL-C < 55mg/dL over time.
The percentage of patients with LDL < 70mg/dL and LDL-C < 55mg/dL are shown for each year assessed and for all 3 years combined. A significant improvement in LDL-C control is seen, but optimal LDL-C goal attainment is still low and with high individual variability over years and difficulties to keep LDL-C persistently below target. LDL, low-density lipoprotein; LDL-C, low-density lipoprotein cholesterol.
Our results show that LDL-C control in a very high-risk outpatient population was poor in 2013 and is still low in 2018. There is still a little achievement of recommended LDL-C target, with only a quarter and a third of patients with an LDL-C < 70mg/dL in 2013 and 2018, respectively, and only 8% and 11.7% of patients with an LDL-C < 55mg/dL in the same years. Patients with an LDL-C ≥ 100mg/dL, a cut off where an important increase in MACE has been observed and where combined lipid-lowering therapy may be more useful,1 still remained around 20.5% in 2018.
These data are in concordance with multicentric registries.13,14,16,18,20 EUROASPIRE V, an international registry conducted among 27 European countries in 2016–2017 and including patients with coronary artery disease, showed that only 29% of them had an LDL-C < 70mg/dL. Among the very high-risk patients from the DYSIS I study, only 21.5% had an LDL-C < 70mg/dL. DYSIS II registry also carried out among several countries in Europe, Asia, and the Middle East between 2012 and 2014, showed that among patients with stable coronary artery disease only 28.3% of patients had an LDL-C < 70mg/dL. In the REPAR Spanish registry, which included patients with very high cardiovascular risk from 2013 to 2014, 26.4% of the cohort had an LDL-C < 70mg/dL. A recent follow-up study including patients with coronary artery bypass graft surgery in Israel showed that 44% of patients had an LDL < 70mg/dL, although the year of data collection is not described.23 In almost all of the registries mentioned, a vast majority of patients (80–90%) are reported to be receiving some dose of statin. Given that impact on cardiovascular outcomes arises from LDL-C reduction independently of the treatment used, our aim was to assess individual trends in LDL-C variation and goal attainments in LDL-C control irrespective of the drugs used to achieve it, so we did not collect data on the treatment used.
Individual trends in LDL-CWhen looking at the temporal trend, a better LDL-C control was achieved along the years, with a median 6mg/dL decrease from 2013 to 2018, a 9.2% absolute increase in the percentage of patients with an LDL-C < 70mg/dL. To our knowledge, this is the first register reporting longitudinal LDL-C variation from the same cohort in the recent years, since many of the published data comes from studies reporting transversal LDL-C levels in a selected time period.
The increase in the percentage of patients with LDL-C < 70mg/dL and LDL-C < 55mg/dL in 2018 is probably related to the release of the aforementioned clinical trials and the spreading notion of “the lower the better” when targeting LDL-C. Although we have not specifically assessed which treatment was used in every patient, there was no restriction in lipid-lowering drugs prescription in our area. Possibly, this improvement reflects the increased risk perception physicians have regarding their patients and their efforts to lower LDL-C by any means, not only by prescribing non-statin drugs but also by intensifying statin treatment.
When assessing maintenance of LDL-C at the desired target, it is remarkable that only 10.0% and 1.38% of patients had all LDL-C measurements < 70mg/dL and < 55mg/dL, respectively. In a similar way, 47.4% and 78.8% had all 3 LDL-C measurements ≥ 70 and 55mg/dL, respectively, male sex and diabetes being significant predictors for this endpoint after adjusting for covariates with a strong odds ratio. This reflects that there is a high prevalence of patients who do not reach LDL-C target at any time and may be exposed to greater risk.
Clinical predictors for achieving optimal LDL-C controlIn all pivotal trials assessing lipid-lowering drugs in secondary prevention,7–9,11,12 no subgroup effect depending on sex or baseline risk factor has been identified in LDL-C reduction, except for a greater reduction in LDL-C in diabetic patients with ezetimibe.10 Previous registries have shown that LDL-C lowering therapy is intensified and better LDL-C control is achieved in groups that are probably judged by the treating physician as being at higher risk for MACE,18,22,24 even when no difference between groups in LDL-C goal is made by ESC and ACC/AHA guidelines when cardiovascular risk is so high.4,5 This may explain the fact that male sex, diabetes or hypertension showed in our study an association with better LDL-C control, since the treating physician may consider these subgroups as being at higher risk for recurrent MACE.
Despite having been an increase in LDL-C target attainment from 2013 to 2018, male sex and diabetes remained predictors for adequate LDL-C control in 2018, that may reflect the fact that risk perception of these specific groups have remained unchanged from 2013 to 2018 ?physicians may consider male or diabetic patients as being exposed to higher risk for recurrent MACE both in 2013 and in 2018.
No subgroup has obtained a specific benefit in terms of LDL-C levels along the assessed years, given that improvement has been due to better LDL-C levels in all patients in the cohort. These data are in concordance with the hypothesis that recent scientific literature may have boosted a change in risk perception globally among treating physicians that has affected equally all subgroups of patients.
It is well known that male sex is a risk factor for developing coronary heart disease, but once it is established, no difference in risk for recurrent events should be made based on sex. This fact may wrongly influence the propensity of the treating physician when adjusting lipid-lowering drugs and based on patient sex,25 which may lead to different outcomes in the short26–28 and in the long term,29–32 although little is known about the correlation of proper LDL-C control, drug prescription and recurrent events in this population. We consider that the present data should warn us against instinctive, non-evidenced based risk perception and should prompt further research to clarify the reasons for a worse LDL-C control in women and non-diabetics. Nonetheless, a systematic review points to a better statin adherence in primary prevention in male patients and diabetes, a fact that may also explain our findings.33
Targeting LDL-C < 55mg/dLWe present the first registry to show data regarding the new target set by 2019 ESC guidelines. Although it was not the aim of the treating physician to lower LDL-C levels below 55 mg/dL, only a minority of patients reached an LDL-C < 55mg/dL despite being at very high risk. We have no data on drug use, but regardless of the medications used on our patients, it is certain that a very important treatment intensification will be necessary to reach the new target, so new studies considering drug prescription and cost-effectiveness should be conducted to assess the feasibility of lowering LDL-C < 55mg/dL.
Strengths and limitationsThis study has several limitations. Because of a retrospective design, we could not control all inherent biases. Patients with higher LDL-C may have a higher mortality rate and we may be overestimating the impact of LDL-C reduction. We excluded 204 patients because there was no LDL-C available in at least one of the selected years. It could be considered that patients with 3 LDL-C measurements available are probably better controlled than patients with no LDL-C measured in any of the 3 selected years. Nevertheless, LDL-C attainment is still poor in this cohort with theoretically better LDL-C control.
All patients included in our study came from a single primary healthcare network in Spain, and results may not be inferred to other European countries, given regional differences seen in multinational registries as EUROASPIRE IV and V.13,20 Also, since we have only selected patients diagnosed with MI at least 5 years before 2018 and with out-of-hospital follow-up, our data may not be extrapolated to patients with recent MI or ischemic heart disease without MI.
When assessing clinical predictors we did not include in our analysis chronic kidney disease or current smoking, both recently associated with better LDL-C control,18 but seems unlikely that its inclusion would have modified our results when, in the same study, both male sex and diabetes remained independent predictors for an LDL-C below target in the single year assessed when multivariate analysis was performed.
Also, given that we have not collected clinical outcomes, one might think that patients considered as being at higher risk, such as male or diabetic patients, may have had more MI during follow-up and lipid-lowering therapy may have been adjusted after the new MI. However, since there were no differences in the prevalence of LDL-C below target in 2013 between patients with a MI in 2013 and patients with a MI before 2013, we consider that the effect of a new MI in LDL-C reduction in patients with already known coronary heart disease is probably not significant. Instead, we believe that better LDL-C control is attained in this population because of a different perception in risk by the treating physician.
ConclusionsIn 2018, only one third and one tenth of the patients with previous MI reach LDL-C < 70mg/dL and 55mg/dL, respectively, although LDL-C control improved significantly from 2013 to 2018. Moreover, only in very few patients LDL-C is maintained below target over the years, male sex and diabetes being clinical predictors for it.
A small proportion of patients with previous MI achieved the ACC/AHA and previous ESC goal of LDL-C < 70mg/dL. New ESC guidelines recommend LDL-C < 55mg/dL as the new target. Nowadays, the percentage of patients with an LDL-C < 55mg/dL is unknown.
The impact on real life population of recent clinical trials reporting the benefit of lowering LDL-C further has not yet been assessed.
Although several clinical predictors have been described for LDL-C < 70mg/dL in a single measurement, no clinical predictors for maintaining an LDL-C below target over time have been assessed.
Does it contribute anything new?This is the first large-scale registry reporting LDL-C control in 2018 and individual LDL-C variation in the last years in very high-risk patients.
LDL-C control and goal attainment has improved in the past 5 years, but in 2018 only a third of patients achieved an LDL-C < 70mg/dL and just 11.7% an LDL < 55mg/dL, so treatment will have to be intensified to reach the new target in almost all patients.
Male sex and diabetic patients achieved in a higher proportion a maintained LDL < 70mg/dL, possibly reflecting differences in risk perception, treatment prescription or treatment adherence.
Funding was provided by FEDER (European Regional Development Fund grants).
Conflicts of interestJ.A Barrabés reports personal fees from AstraZeneca, and from Bayer, outside the submitted work. G. Oristrell reports personal fees from AstraZeneca, from Pfizer, from Merck Sharp and Dohme, and from Daiichi Sankyo, outside the submitted work.
All authors are supported by the Spanish Health Institute and CIBERCV.