To assess mortality, cardiovascular and renal outcomes among patients with heart failure (HF) (primary objective), with a particular focus on the risk of developing chronic kidney disease (CKD) (secondary).
MethodsObservational cohort study, comprising cross-sectional and longitudinal retrospective analyses using secondary data from electronic health records. For the primary objective, adults with prevalent HF, defined as at least one diagnosis of HF prior to the index date (1 January 2017) were included. For the secondary objective, adults with incident HF in 2017 were enrolled.
ResultsA total of 21 575 patients had HF in the prevalent population (8391 with CKD at baseline), whereas 3045 patients were included in the incident population. In the prevalent population, the risk of all-cause death (HR, 1.227; 95%CI, 1.172–1.285), CKD hospitalization (HR, 1.427; 95%CI, 1.379–1.479) and acute kidney failure (HR, 1.377; 95%CI, 1.222–1.524) was greater in those patients with HF and CKD vs HF only after 3 years of follow-up. For the incident population, within 24 months from HF diagnosis, 5.9% of patients developed CKD. Overall, 23.4% were taking angiotensin-converting enzyme inhibitors, 26.3% angiotensin receptor blockers, 7.9% sacubitril/valsartan, 64.2% beta blockers, 11.5% aldosterone antagonists and 4.5% sodium-glucose Cotransporter-2 inhibitors.
ConclusionsIn Spain, patients with HF have a high risk of developing cardiovascular and renal complications. Despite that, there is a substantial proportion of patients that are not taking guideline recommended drugs. A higher use of these drugs could reduce HF burden and complications in clinical practice.
Determinar la mortalidad y los eventos cardiovasculares y renales en pacientes con insuficiencia cardiaca (IC) (objetivo primario), en particular sobre el desarrollo de enfermedad renal crónica (ERC) (secundario).
MétodosEstudio observacional, con análisis transversal y retrospectivo, empleando datos secundarios de registros electrónicos de salud. Para el objetivo primario se incluyeron adultos con IC prevalente, definida como al menos un diagnóstico de IC antes de la fecha índice (1 de enero de 2017). Para el secundario se incluyeron adultos con IC incidente en 2017.
ResultadosSe incluyeron 21.575 pacientes con IC en la población prevalente (8.391 con ERC basal) y 3.045 en la población incidente. En la población prevalente el riesgo de muerte (HR=1,227; IC95%, 1,172-1,285), hospitalización por ERC (HR=1,427; IC95%, 1,379-1,479) y fallo renal agudo (HR=1,377; IC95%, 1,222-1,524) fue mayor en los pacientes con IC y ERC frente a IC sola, tras 3años de seguimiento. En la población incidente, a los 24meses del diagnóstico el 5,9% desarrollaron ERC. Globalmente, el 23,4% tomaban inhibidores de la enzima convertidora de angiotensina, el 26,3% antagonistas de los receptores de angiotensinaII, el 7,9% sacubitrilo/valsartán, el 64,2% bloqueadores beta, el 11,5% antialdosterónicos y el 4,5% inhibidores del cotransportador sodio-glucosa tipo2.
ConclusionesEn España, los pacientes con IC tienen un riesgo elevado de desarrollar complicaciones cardiovasculares y renales. Sin embargo, existe una proporción importante de pacientes que no toman los fármacos recomendados por las guías. Un mayor uso podría reducir la carga de IC y las complicaciones en la práctica clínica.
Heart failure (HF) is a common condition. It has been estimated that the current prevalence reaches 2% in developed countries, but will increase in the following years due to the ageing of the population, better management of acute cardiovascular conditions and new HF treatments.1–4 Despite traditional HF therapies, HF is associated with a poor prognosis and high cost burden.5,6 In fact, in 2013, the MAGGIC meta-analysis reported that around 40% of HF patients died after only 2.5 years of follow-up.7 Hospitalizations are the most common complication in patients with chronic HF, mainly due to acute HF decompensation, but also because other conditions, such as chronic kidney disease (CKD).4,8–10
The 2021 European HF guidelines recommend the use of angiotensin-converting enzyme inhibitors or sacubitril/valsartan, beta blockers, aldosterone antagonists, and sodium-glucose Cotransporter-2 (SGLT-2) inhibitors as first-line therapies in patients with reduced HF and should be early used in this population.1 However, according to the available evidence,11,12 the 2016 HF guidelines recommended a step by step approach, starting with the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and beta blockers as a first-line approach, adding aldosterone antagonists if symptoms persisted and then changing to sacubitril/valsartan from angiotensin-converting enzyme inhibitors/angiotensin receptor blockers or adding ivabradine if patients remained symptomatic.13 Of note, although clinical trials performed in diabetic population had suggested a positive effect on HF incidence with the use of some SGLT-2 inhibitors,12 their beneficial effects in patients with reduced HF, regardless diabetes status, has recently been demonstrated.14,15 Unfortunately, data regarding the impact of these new therapies on morbidity and mortality in real-life patients are lacking. On the other hand, patients with reduced ejection fraction exhibit relevant disparities in the clinical profile, treatment and prognosis compared to patients with preserved ejection fraction.16–18 However, current data about differences in the management and outcomes of both entities are scarce.
The objectives of this study were to assess all-cause mortality, and cardiovascular and renal outcomes among patients with HF (primary objective), with a particular focus on the risk of developing CKD following diagnosis of HF (secondary objective).
MethodsObservational and cohort study, using secondary data collected from the electronic health records of 7 Spanish Autonomous Communities within the validated BIG PAC database.19 This study was approved by the Investigation Ethics Committee of Consorci Sanitari from Terrassa. No informed consent was provided, as this was a secondary data study and data were fully anonymized and dissociated from patients.
To assess the objectives of the study, cross-sectional and longitudinal retrospective analyses were performed. For the primary objective of the study, adults with prevalent HF, defined as at least one diagnosis of HF prior to the index date (1 January 2017) (prevalent population) were included. CKD was defined as an estimated glomerular filtration rate (eGFR)<60mL/min/1.73m2 by CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) or ≥60mL/min/1.73m2 with a urine albumin-to-creatinine ratio (UACR) ≥30mg/g at index date. For the secondary objective, adults with incident HF, defined as new diagnosis of HF in 2017 were included (incident population). Index date was the first HF diagnosis date in 2017. According to DAPA-HF and DELIVER trials,14,20 HF with reduced o preserved left ventricular ejection fraction was defined as those patients with HF and a left ventricular ejection fraction≤40% or >40%, respectively. In both populations, CKD stages were classified, as follows: CKD stage 1: eGFR≥90mL/min/1.73m2 and UACR≥30mg/g; CKD stage 2: eGFR 60–89mL/min/1.73m2 and UACR≥30mg/g; CKD stage 3a: eGFR 45–59mL/min/1.73m2; CKD stage 3b: eGFR 30–44mL/min/1.73m2; CKD stage 4: eGFR 15–29mL/min/1.73m2; CKD stage 5: eGFR<15mL/min/1.73m2; CKD unspecified: no eGFR data available.
In the prevalent population, at baseline (index date 1 January 2017), biodemographic data, physical examination, HF data, laboratory data, comorbidities and concomitant drugs were recorded. In the incident population, baseline clinical characteristics, including comorbidities, laboratory data and concomitant medications were reported in relation to the index date (the first HF diagnosis date in 2017). Data were presented according to the HF type and CKD stage.
Regarding the primary objective (prevalent population), for overall mortality, patient follow-up began at index date (1 January 2017) and continued until the death date or censored at the earliest of the end of enrolment for the latest available linked data or observational study period end date (31 December 2019, 3 years of follow-up). For other outcomes, patient follow-up began on the index date and continued until the specified cardiorenal event (hospitalization for HF, CKD, albuminuria transition from UACR<30 to 30–300mg/g and acute kidney failure) occurred or was censored at the earliest of the end of enrolment for the latest available linked data, death date or observational study period end date (31 December 2019, 3 years of follow-up). Within each event category, patients were censored after the first event for the category but not for events from other categories. For the secondary objective of the study, patients were followed from HF diagnosis in 2017, for 24 months.
Statistical analysisAbsolute and relative frequency distributions were used to describe the qualitative variables and mean and standard deviation for quantitative variables. Event rates were calculated as the number of new cases from index date in the 24 months of follow up divided by the total time at risk of the event. Event rates were presented as events and events per 100 patient-years for all-cause death, HF, CKD, and albuminuria. Time to first hospitalization due to event was analyzed descriptively. Follow-up was censored at observation period, or death end unless an event had occurred. The corresponding adjusted hazard ratios and 95% confidence intervals to estimate the risk of outcomes in the prevalent population after 3 years of follow-up were calculated. The pathway to develop CKD in patients with incident HF was evaluated for 24 months from index date. Categorical variables were compared using the chi-square test or the Fisher exact test when appropriate. When 2 means were compared, the t test or the Mann–Whitney test was used, as applicable. The data were analyzed using the statistical package SPSS v25.0 (SPSS Inc., Chicago, United States).
ResultsOverall, 21 575 patients had HF in the prevalent population (13 184 without CKD at baseline and 8391 with CKD at baseline), whereas 3045 patients were included in the incident population (Fig. 1 of the supplementary data).
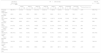
Among patients with HF but without CKD at baseline, mean age was 77.8±13.7 years, 52.7% were men, 31.9% type 2 diabetes, and 21.4% prior myocardial infarction. With regard to the type of HF, 51.7% had HF with reduced left ventricular ejection fraction and 48.3% HF with preserved left ventricular ejection fraction. With respect to treatments, 30.6%, 32.4% and 7.7% were taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and sacubitril/valsartan, respectively, and 4.3% SGLT-2 inhibitors. Compared with patients with HF without CKD, those with CKD were older, more commonly women, had higher levels of systolic blood pressure, body mass index, glycosylated hemoglobin (HbA1c), UACR and serum potassium levels. Comorbidities, including diabetes, atrial fibrillation, myocardial infarction, stroke, and peripheral artery disease were more common in those patients with CKD and HF (vs HF alone). In addition, more patients in the HF and CKD population (vs HF alone) were taking more angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and beta blockers, with a similar proportion of aldosterone antagonists and SGLT-2 inhibitors (Table 1).
Clinical characteristics and treatments in the prevalent HF population at index date.
| Only HF(n=13 184; 61.1%) | HF+CKD(n=8391; 38.9%) | PHF+CKDvsCKD | |
|---|---|---|---|
| Biodemographic data | |||
| Age, years | 77.8±13.7 | 79.4±10.9 | <.001 |
| Gender, male, (%) | 6949 (52.7) | 4237 (50.5) | <.001 |
| Physical examination | |||
| Systolic blood pressure, mmHg | 130.7±21.3 | 133.9±20.5 | <.001 |
| Diastolic blood pressure, mmHg | 83.8±7.2 | 83.7±6.9 | .312 |
| BMI, kg/m2 | 28.4±5.0 | 28.9±5.2 | <.001 |
| BMI>30kg/m2, n (%) | 2914 (22.1) | 2013 (24.0) | <.001 |
| Laboratory data | |||
| HbA1c, % | 7.3±1.8 | 7.7±2.0 | <.001 |
| <7%, n (%) | 6895 (52.3) | 4256 (50.7) | <.001 |
| 7 to <8%, n (%) | 2278 (17.3) | 1680 (20.0) | <.001 |
| 8 to <9%, n (%) | 1185 (9.0) | 867 (10.3) | <.001 |
| ≥9%, n (%) | 936 (7.1) | 769 (9.2) | <.001 |
| eGFR* | 85.5±7.2 | 46.4±9.8 | <.001 |
| UACR, mg/g | 16.7±9.8 | 361.2±148.5 | <.001 |
| Serum potassium levels, mmol/L | 4.5±0.5 | 5.7±1.6 | <.001 |
| Left ventricular ejection fraction, % | 44.2±10.2 | 43.4±10.1 | .235 |
| Comorbidities,n(%) | |||
| CVD | 5415 (41.1) | 5180 (61.7) | <.001 |
| Stroke | 1364 (10.3) | 1030 (12.3) | <.001 |
| Myocardial infarction | 2824 (21.4) | 2154 (25.7) | <.001 |
| PAD | 681 (5.2) | 564 (6.7) | <.001 |
| Atrial fibrillation | 4379 (33.2) | 2970 (35.4) | <.001 |
| HF | 13 184 (100) | 8391 (100) | – |
| HF-reduced ejection fraction | 6810 (51.7) | 4465 (53.2) | <.001 |
| HF-preserved ejection fraction | 6374 (48.3) | 3926 (46.8) | <.001 |
| CKD | – | 8391 (100) | – |
| Stage 1 | – | 977 (11.6) | – |
| Stage 2 | – | 1584 (18.9) | – |
| Stage 3a | – | 1753 (20.9) | – |
| Stage 3b | – | 1961 (23.4) | – |
| Stage 4 | – | 1127 (13.4) | – |
| Stage 5 | – | 296 (3.5) | – |
| Type 2 diabetes | 4200 (31.9) | 5034 (60.0) | <.001 |
| Hyperkalemia | 6 (0) | 851 (10.1) | – |
| Medications, n (%) | |||
| CVD risk treatment | 13 184 (100) | 8391 (100) | – |
| Antihypertensives | 12 230 (92.8) | 7960 (94.9) | <.001 |
| ACEi | 4034 (30.6) | 2716 (32.4) | <.001 |
| ARBs | 4269 (32.4) | 3548 (42.3) | <.001 |
| ARNI | 1017 (7.7) | 743 (8.9) | .142 |
| Beta blockers | 9107 (69.1) | 5998 (71.5) | <.001 |
| Loop diuretics | 9263 (70.3) | 5978 (71.2) | .186 |
| Aldosterone antagonists | 4258 (32.3) | 2781 (33.1) | .130 |
| Calcium channel blockers | 1234 (9.4) | 658 (7.8) | <.001 |
| Thiazide diuretics | 665 (5.0) | 433 (5.2) | .747 |
| Antidiabetics | 3823 (29.0) | 3571 (42.6) | <.001 |
| Metformin | 2739 (20.8) | 2021 (24.1) | <.001 |
| Sulfonylurea | 1571 (11.9) | 969 (11.5) | .761 |
| DPP4 inhibitors | 1371 (10.4) | 962 (11.5) | .098 |
| SGLT-2 inhibitors | 571 (4.3) | 333 (4.0) | .933 |
| GLP-1 receptor agonists | 141 (1.1) | 221 (2.6) | <.001 |
| Metiglinides | 190 (1.4) | 375 (4.5) | <.001 |
| Thiazolidinediones | 25 (0.2) | 27 (0.3) | .977 |
| Acarbose | 22 (0.2) | 20 (0.2) | .849 |
| Insulin | 950 (7.2) | 1255 (15.0) | <.001 |
| Statins | 7846 (59.5) | 5327 (63.5) | <.001 |
| Digoxin | 878 (6.7) | 524 (6.2) | .891 |
| Nitrates | 1442 (10.9) | 1267 (15.1) | <.001 |
| Warfarin/acenocoumarol | 2506 (19.0) | 1887 (22.5) | <.001 |
| Low dose aspirin | 2819 (21.4) | 2518 (30.0) | <.001 |
| Receptor P2Y12antagonists | 1041 (7.9) | 880 (10.5) | <.001 |
ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ARNI, angiotensin receptor and neprilysin inhibition; BMI, body mass index; CVD, cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DPP4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; *, mL/min/1.73m2; GLP-1, glucagon-like peptide-1; HF, heart failure; PAD, peripheral artery disease; SGLT-2, sodium-glucose Cotransporter-2; UACR, Urine albumin-to-creatinine ratio; hyperkalemia: serum potassium>5.5mmol/L.
Regarding outcomes between HF vs HF and CKD patients in the prevalent population after 3 years of follow-up, the risk of all-cause death (HR, 1.227; 95%CI, 1.172–1.285; P<.001), hospitalization for CKD (HR, 1.427; 95%CI, 1.379–1.479; P<.001), and acute kidney failure (HR, 1.377; 95%CI, 1.222–1.524; P<.001) was greater in those patients with HF and CKD compared to HF only patients (Table 2, and Fig. 2 of the supplementary data).
Risk of outcomes* between HF vs CKD and HF patients in the prevalent population after 3 years of follow-up.
| Group | Endpoint | Follow-up (median, days) | Events, N | % | HRCKDandHFvsHF | 95%CI | P |
|---|---|---|---|---|---|---|---|
| CKD and HF | All-cause death | 428 | 3132 | 37.3% | 1.227 | 1.172–1.285 | <.001 |
| HF | 552 | 4579 | 34.7% | ||||
| CKD and HF | HF | 447 | 3994 | 47.6% | 1.013 | 0.973–1.056 | .526 |
| HF | 458 | 5416 | 41.1% | ||||
| CKD and HF | CKD | 545 | 2097 | 25.0% | 1.427 | 1.379–1.479 | <.001 |
| HF | 538 | 3070 | 23.3% | ||||
| CKD and HF | UACR progression: <30 to 30–300mg/g | 504 | 43 | 0.5% | 1.309 | 0.956–1.770 | .094 |
| HF | 548 | 725 | 5.5% | ||||
| CKD and HF | Acute kidney failure (ICD N17) | 592 | 164 | 2.0% | 1.377 | 1.222–1.524 | <.001 |
| HF | 642 | 49 | 0.4% |
95%CI, confidence interval; CKD, chronic kidney disease; HF, heart failure; UACR, urine albumin-to-creatinine ratio.
* All-cause mortality and first hospitalization for cardiorenal events (HF, CKD, acute kidney failure) or albuminuria transitions during follow-up.
In the incident population, 3045 patients with HF (2435 [79.6%] without CKD at baseline) were included (Table 3). Overall, mean age was 65.4±23.2 years, 51.4% men, left ventricular ejection fraction 45.4±11%, eGFR 85.8±7.9mL/min/1.73m2 and UACR 96.5±44.4mg/g. One third of patients had diabetes, 20.0% CKD and 14.1% prior myocardial infarction. Patients without CKD at baseline were younger and suffered less from prior myocardial infarction, atrial fibrillation or stroke. Data according to the type of HF (reduced and preserved left ventricular ejection fraction) are shown in Tables 1 and 2 of the supplementary data. Despite patients with preserved HF were older than those with reduced HF, both had many comorbidities. With regard to HF treatments, in the overall incident HF population, 23.4% were taking angiotensin-converting enzyme inhibitors, 26.3% angiotensin receptor blockers, 7.9% sacubitril/valsartan (total 57.6%), 64.2% beta blockers, 11.5% aldosterone antagonists and 4.5% SGLT-2 inhibitors.
Clinical characteristics and treatments in the incident HF population at baseline.
| All HF (n=3045; 100%) | HF without CKD (n=2435; 79.6%) | PHFwithoutCKDvsHFCKD | HF+CKD at index date | Total with CKD (n=610; 20.4%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 (n=19; 3.1%) | Stage 2 (n=115; 18.9%) | PStage2vs1 | Stage 3a (n=201; 33.0%) | PStage3avs1 | Stage 3b (n=148; 24.3%) | PStage3bvs1 | Stage 4 (n=44; 7.2%) | PStage4vs1 | Stage 5 (n=37; 6.1%) | PStage5vs1 | Unspecified (n=46; 7.5%) | PUnspvs1 | |||||
| Biodemographic data | |||||||||||||||||
| Age, years | 65.4±23.2 | 60.6±20.4 | <.001 | 58.4±24.5 | 59.8±24.4 | .817 | 61.1±23.6 | .635 | 65.2±22.7 | .225 | 68.7±21.4 | .099 | 69.8±20.3 | .069 | 59.1±20.8 | .907 | 67.8±22.1 |
| Gender, male, n (%) | 1565 (51.4) | 1259 (51.7) | .116 | 5 (26.3) | 58 (50.4) | <.001 | 103 (51.2) | <.001 | 74 (50.0) | <.001 | 23 (52.3) | <.001 | 19 (51.4) | <.001 | 24 (52.2) | <.001 | 306 (50.2) |
| Physical examination | |||||||||||||||||
| Systolic blood pressure, mmHg | 129.8±19.8 | 129.6±20.4 | .595 | 128.6±21.2 | 128.7±21.3 | .798 | 129.2±20.5 | .759 | 129.1±20.6 | .845 | 129.8±20.8 | .756 | 130.2±22.1 | .845 | 128.7±19.7 | .657 | 130.1±22.1 |
| Diastolic blood pressure, mmHg | 83.5±6.9 | 83.4±7.1 | <.001 | 82.9±7.0 | 82.8±6.8 | .854 | 82.9±6.9 | .985 | 83.2±7.0 | .756 | 83.1±7.2 | .654 | 84.1±7.1 | .761 | 82.9±7.0 | .758 | 82.2±6.9 |
| BMI, kg/m2 | 28.7±5.1 | 28.6±5.0 | <.001 | 28.5±4.9 | 28.7±4.8 | .867 | 28.6±4.9 | .854 | 28.8±5.1 | .654 | 28.9±5.0 | .754 | 28.9±4.9 | .752 | 28.7±5.1 | .861 | 27.8±5.0 |
| HF data | |||||||||||||||||
| Left ventricular ejection fraction, % | 45.4±11 | 45.8±9 | <.001 | 45.7±10.6 | 45.5±9.9 | .789 | 45.5±9.1 | .845 | 45.4±10.6 | .745 | 45.3±11 | .845 | 44.9±10.5 | .456 | 45.8±10.6 | .769 | 43.3±9.4 |
| LVEF≤40%, n (%) | 1542 (50.6) | 1223 (50.2) | .155 | 10 (52.6) | 58 (50.4) | .7 | 106 (52.7) | .997 | 79 (53.4) | .829 | 23 (52.3) | .974 | 19 (51.4) | .982 | 24 (52.2) | .8 | 319 (52.3) |
| LVEF>40%, n (%) | 1496 (49.1) | 1205 (49.5) | .159 | 9 (47.4) | 57 (49.6) | .936 | 95 (47.3) | .993 | 69 (46.6) | .762 | 21 (47.7) | .795 | 18 (48.6) | .804 | 22 (47.8) | .796 | 291 (47.7) |
| NYHA functional class, n (%) | |||||||||||||||||
| I | 386 (12.7) | 311 (12.8) | .829 | 3 (15.8) | 15 (13.0) | .987 | 26 (12.9) | .715 | 17 (11.5) | .717 | 4 (9.1) | .63 | 3 (8.1) | .848 | 7 (15.2) | .933 | 75 (12.3) |
| II | 1391 (45.7) | 1130 (46.4) | <.001 | 8 (42.1) | 48 (41.7) | .996 | 87 (43.3) | .873 | 63 (42.6) | .982 | 19 (43.2) | .872 | 16 (43.2) | .765 | 20 (43.5) | .802 | 261 (42.8) |
| III | 1134 (37.2) | 890 (36.6) | <.001 | 7 (36.8) | 44 (38.3) | .797 | 80 (39.8) | .85 | 61 (41.2) | .862 | 19 (43.2) | .837 | 16 (43.2) | .79 | 17 (37.0) | .84 | 244 (40.0) |
| IV | 124 (4.1) | 97 (4.0) | .798 | 1 (5.3) | 4 (3.5) | .801 | 9 (4.5) | .912 | 7 (4.7) | .706 | 2 (4.5) | .777 | 2 (5.4) | .954 | 2 (4.3) | .842 | 27 (4.4) |
| Laboratory data | |||||||||||||||||
| eGFR* | 85.8±7.9 | 96.2±4.9 | <.001 | 94.3±3.7 | 74.7±8.6 | <.001 | 52.1±10.8 | <.001 | 36.8±10.6 | <.001 | 21.9±10.5 | <.001 | 8.8±7.4 | <.001 | – | – | 43.4±8.8 |
| UACR, mg/g | 96.5±44.4 | 14.8±6.8 | <.001 | 105.8±43.4 | 128.1±64.1 | <.001 | 249.8±122.4 | <.001 | 261.3±109.7 | <.001 | 1631.4±750.4 | <.001 | 1651.3±726.6 | <.001 | 122.3±51.4 | <.001 | 384.2±180.6 |
| HbA1c. % | 7.1±1.9 | 5.3±1.4 | <.001 | 6.1±1.7 | 6.8±2 | .152 | 6.9±1.9 | <.001 | 7.1±2 | .421 | 7.3±2.1 | .345 | 7.5±2 | .453 | 6.9±2 | .453 | 6.7±1.9 |
| <7%, n (%) | 1574 (51.7) | 1256 (51.6) | .924 | 10 (52.6) | 60 (52.2) | .949 | 105 (52.2) | .907 | 77 (52.0) | .986 | 23 (52.3) | .912 | 19 (51.4) | .703 | 24 (52.2) | .779 | 318 (52.1) |
| 7–<8%, n (%) | 535 (17.6) | 433 (17.8) | .627 | 0 | 19 (16.5) | – | 35 (17.4) | – | 26 (17.6) | – | 7 (15.9) | .279 | 7 (18.9) | .075 | 8 (17.4) | .183 | 102 (16.7) |
| 8–<9%, n (%) | 270 (8.9) | 205 (8.4) | <.001 | 2 (10.5) | 11 (9.6) | .893 | 20 (10.0) | .784 | 18 (12.2) | .815 | 5 (11.4) | .84 | 5 (13.5) | .844 | 4 (8.7) | .851 | 65 (10.7) |
| ≥9%, n (%) | 231 (7.6) | 174 (7.1) | .074 | 1 (5.3) | 9 (7.8) | .82 | 19 (9.5) | .989 | 16 (10.8) | .566 | 5 (11.4) | .78 | 4 (10.8) | .946 | 3 (6.5) | .761 | 57 (9.3) |
| Comorbidities,n(%) | |||||||||||||||||
| CVD | 2246 (73.8) | 1801 (74.0) | .374 | 14 (73.7) | 85 (73.9) | .927 | 148 (73.6) | .81 | 105 (70.9) | .890 | 32 (72.7) | .72 | 28 (75.7) | .907 | 33 (71.7) | .927 | 445 (73.0) |
| Myocardial infarction | 429 (14.1) | 307 (12.6) | <.001 | 3 (15.8) | 23 (20.0) | .98 | 41 (20.4) | .998 | 29 (19.6) | .959 | 9 (20.5) | .765 | 8 (21.6) | .818 | 9 (19.6) | .812 | 122 (20.0) |
| Stroke | 183 (6.0) | 101 (4.1) | <.001 | 2 (10.5) | 14 (12.2) | .988 | 27 (13.4) | .9 | 23 (15.5) | .488 | 6 (13.6) | .968 | 5 (13.5) | .769 | 5 (10.9) | .751 | 82 (13.4) |
| Atrial fibrillation | 836 (27.5) | 626 (25.7) | <.001 | 7 (36.8) | 39 (33.9) | .891 | 69 (34.3) | .961 | 52 (35.1) | .925 | 15 (34.1) | .995 | 13 (35.1) | .942 | 15 (32.6) | .871 | 210 (34.4) |
| Peripheral artery disease | 137 (4.5) | 102 (4.2) | .243 | 1 (5.3) | 6 (5.2) | .923 | 11 (5.5) | .729 | 10 (6.8) | .965 | 3 (6.8) | .94 | 2 (5.4) | .849 | 2 (4.3) | .916 | 35 (5.7) |
| CKD | 610 (20.0) | 0 | – | 19 (100) | 115 (100) | – | 201 (100) | – | 148 (100) | – | 44 (100) | – | 37 (100) | – | 46 (100) | – | 610 (100) |
| Diabetes | 1010 (33.2) | 801 (32.9) | .241 | 7 (36.8) | 38 (33.0) | .766 | 70 (34.8) | .953 | 51 (34.5) | .894 | 15 (34.1) | .754 | 13 (35.1) | .873 | 15 (32.6) | .898 | 209 (34.3) |
| Medications,n(%) | |||||||||||||||||
| HF medication | 3001 (98.6) | 2391 (98.2) | .206 | 19 (100) | 115 (100) | – | 201 (100) | – | 148 (100) | – | 44 (100) | – | 37 (100) | – | 46 (100) | – | 610 (100) |
| RAAS inhibitors | 1493 (49.0) | 1205 (49.5) | <.001 | 10 (52.6) | 51 (44.3) | .205 | 93 (46.3) | .506 | 70 (47.3) | .492 | 21 (47.7) | .933 | 19 (51.4) | .98 | 24 (52.2) | .919 | 288 (47.2) |
| ACEi | 712 (23.4) | 558 (22.9) | <.001 | 5 (26.3) | 27 (23.5) | .986 | 50 (24.9) | .847 | 37 (25.0) | .892 | 12 (27.3) | .77 | 11 (29.7) | .986 | 12 (26.1) | .911 | 154 (25.2) |
| ARBs | 801 (26.3) | 632 (26.0) | .128 | 6 (31.6) | 30 (26.1) | .629 | 54 (26.9) | .729 | 42 (28.4) | .843 | 12 (27.3) | .785 | 11 (29.7) | .968 | 14 (30.4) | .74 | 169 (27.7) |
| Beta blockers | 1954 (64.2) | 1531 (62.9) | <.001 | 13 (68.4) | 80 (69.6) | .733 | 138 (68.7) | .74 | 103 (69.6) | .934 | 31 (70.5) | .894 | 26 (70.3) | .958 | 32 (69.6) | .767 | 423 (69.3) |
| Loop-diuretics | 2090 (68.6) | 1655 (68.0) | <.001 | 13 (68.4) | 83 (72.2) | .937 | 145 (72.1) | .885 | 105 (70.9) | .99 | 31 (70.5) | .774 | 27 (73.0) | .793 | 31 (67.4) | .923 | 435 (71.3) |
| Aldosterone antagonists | 349 (11.5) | 273 (11.2) | .231 | 2 (10.5) | 14 (12.2) | .823 | 25 (12.4) | .828 | 20 (13.5) | .97 | 5 (11.4) | .865 | 4 (10.8) | .891 | 6 (13.0) | .966 | 76 (12.5) |
| ARNI | 242 (7.9) | 187 (7.7) | .275 | 1 (5.3) | 10 (8.7) | .754 | 18 (9.0) | .833 | 15 (10.1) | .946 | 4 (9.1) | .956 | 3 (8.1) | .972 | 4 (8.7) | .791 | 55 (9.0) |
| Digoxin | 213 (7.0) | 169 (6.9) | .866 | 1 (5.3) | 8 (7.0) | .955 | 15 (7.5) | .909 | 11 (7.4) | .73 | 3 (6.8) | .947 | 3 (8.1) | .716 | 3 (6.5) | .722 | 44 (7.2) |
| Antidiabetics | 867 (28.5) | 682 (28.0) | <.001 | 6 (31.6) | 35 (30.4) | .808 | 60 (29.9) | .799 | 45 (30.4) | .962 | 14 (31.8) | .814 | 12 (32.4) | .954 | 13 (28.3) | .838 | 185 (30.3) |
| Metformin | 594 (19.5) | 462 (19.0) | <.001 | 5 (26.3) | 24 (20.9) | .542 | 43 (21.4) | .904 | 32 (21.6) | .865 | 9 (20.5) | .77 | 9 (24.3) | .738 | 10 (21.7) | .987 | 132 (21.6) |
| Sulfonylurea | 367 (12.1) | 295 (12.1) | .774 | 2 (10.5) | 15 (13.0) | .714 | 23 (11.4) | .833 | 17 (11.5) | .892 | 5 (11.4) | .746 | 4 (10.8) | .723 | 6 (13.0) | .746 | 72 (11.8) |
| DPP4 inhibitors | 339 (11.1) | 273 (11.2) | .862 | 2 (10.5) | 12 (10.4) | .703 | 22 (10.9) | .984 | 16 (10.8) | .932 | 5 (11.4) | .909 | 4 (10.8) | .981 | 5 (10.9) | .723 | 66 (10.8) |
| SGLT-2 inhibitors | 138 (4.5) | 101 (4.1) | .236 | 1 (5.3) | 6 (5.2) | .994 | 13 (6.5) | .967 | 10 (6.8) | .832 | 3 (6.8) | .967 | 2 (5.4) | .932 | 2 (4.3) | .87 | 37 (6.1) |
| GLP-1 receptor agonists | 29 (1.0) | 23 (0.9) | .913 | 0 | 0 | – | 1 (0.5) | – | 2 (1.4) | – | 1 (2.3) | – | 1 (2.7) | – | 1 (2.2) | – | 6 (1.0) |
| Metiglinides | 43 (1.4) | 35 (1.4) | .963 | 0 | 1 (0.9) | – | 2 (1.0) | – | 2 (1.4) | – | 1 (2.3) | – | 1 (2.7) | – | 1 (2.2) | – | 8 (1.3) |
| Thiazolidinedione | 10 (0.3) | 5 (0.2) | .714 | 0 | 0 | – | 1 (0.5) | – | 2 (1.4) | – | 1 (2.3) | – | 1 (2.7) | – | 0 | – | 5 (0.8) |
| Acarbose | 6 (0.2) | 5 (0.2) | .973 | 0 | 0 | – | 0 | – | 1 (0.7) | – | 0 | – | 0 | – | 0 | – | 1 (0.2) |
| Insulin | 222 (7.3) | 176 (7.2) | .813 | 1 (5.3) | 8 (7.0) | .763 | 15 (7.5) | .861 | 12 (8.1) | .881 | 4 (9.1) | .823 | 3 (8.1) | .839 | 3 (6.5) | .761 | 46 (7.5) |
| Statins | 1763 (57.9) | 1396 (57.3) | <.001 | 11 (57.9) | 68 (59.1) | .928 | 122 (60.7) | .848 | 90 (60.8) | .786 | 27 (61.4) | .962 | 23 (62.2) | .886 | 26 (56.5) | .735 | 367 (60.2) |
| Antihypertensives | 626 (20.6) | 490 (20.1) | .212 | 4 (21.1) | 24 (20.9) | .900 | 45 (22.4) | .91 | 34 (23.0) | .928 | 10 (22.7) | .848 | 9 (24.3) | .71 | 10 (21.7) | .853 | 136 (22.3) |
| Dihydropyridine CCB | 439 (14.4) | 352 (14.5) | .91 | 2 (10.5) | 17 (14.8) | .997 | 29 (14.4) | .805 | 21 (14.2) | .903 | 6 (13.6) | .898 | 5 (13.5) | .881 | 7 (15.2) | .998 | 87 (14.3) |
| Thiazide diuretics | 185 (6.1) | 143 (5.9) | .421 | 1 (5.3) | 7 (6.1) | .737 | 14 (7.0) | .8 | 11 (7.4) | .896 | 3 (6.8) | .922 | 3 (8.1) | .83 | 3 (6.5) | .747 | 42 (6.9) |
| Non-dihydropyridine CCB | 92 (3.0) | 68 (2.8) | .606 | 1 (5.3) | 4 (3.5) | .945 | 8 (4.0) | .749 | 6 (4.1) | .965 | 2 (4.5) | .763 | 2 (5.4) | .962 | 1 (2.2) | .873 | 24 (3.9) |
| Nitrates | 312 (10.2) | 236 (9.7) | <.001 | 2 (10.5) | 13 (11.3) | .917 | 25 (12.4) | .74 | 19 (12.8) | .968 | 6 (13.6) | .92 | 6 (16.2) | .802 | 5 (10.9) | .831 | 76 (12.5) |
| Warfarin/acenocoumarol | 618 (20.3) | 496 (20.4) | .859 | 3 (15.8) | 22 (19.1) | .902 | 41 (20.4) | .722 | 30 (20.3) | .794 | 9 (20.5) | .836 | 8 (21.6) | .949 | 9 (19.6) | .981 | 122 (20.0) |
| Low dose aspirin | 632 (20.8) | 492 (20.2) | <.001 | 4 (21.1) | 25 (21.7) | .740 | 47 (23.4) | .741 | 35 (23.6) | .831 | 10 (22.7) | .783 | 9 (24.3) | .909 | 10 (21.7) | .803 | 140 (23.0) |
| Receptor P2Y12antagonists | 232 (7.6) | 176 (7.2) | .208 | 1 (5.3) | 9 (7.8) | .813 | 19 (9.5) | .837 | 15 (10.1) | .964 | 4 (9.1) | .835 | 4 (10.8) | .808 | 4 (8.7) | .702 | 56 (9.2) |
ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ARNI, angiotensin receptor and neprilysin inhibition; BMI, body mass index; CCB, calcium channel blockers; CVD, cardiovascular disease; CKD, chronic kidney disease; DPP4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; * mL/min/1.73m2; GLP-1: glucagon-like peptide-1; HF: heart failure; LVEF: left ventricular ejection fraction; RAAS: renin–angiotensin system; SBP: systolic blood pressure; SGLT-2: sodium-glucose cotransporter-2; UACR: urine albumin-to-creatinine ratio.
Within 24 months from HF diagnosis, eGFR progressively decreased from 85.8±7.5 to 75.9±7.3mL/min/1.73m2; P<.001 (from 96.2±3.2 to 85.9±7.8mL/min/1.73m2 among those patients without CKD at baseline), whereas UACR increased from 96.5±44.4 to 103.4±42.4mg/g; P<.001 (from 14.8±6.7 to 15.4±7.2mg/g; P=.003 among those patients without CKD at baseline). The same trend towards worsening was observed in all stages for patients with CKD at baseline (Table 3 of the supplementary data). Thus, during this period, 5.9% of patients developed CKD, regardless of left ventricular ejection fraction (Table 4). In the overall study population, all-cause death, and hospitalization for HF and CKD were 17.2, 20.6 and 9.4 per 100 patient-year, after 24 months of follow-up, respectively. These numbers were 16.1, 20.2 and 9.3 per 100 patient-year in those patients without CKD at baseline and 21.9, 23.8 and 11.0 per 100 patient-year, in those with CKD at baseline, respectively. In general, outcome rates increased and time to first event shortened as renal function worsened (Table 5). These figures were higher in those patients with HF and reduced left ventricular ejection fraction than in those with HF and preserved left ventricular ejection fraction, both in patients with or without CKD at index date, consistent with the slightly higher percentage of patients with reduced ejection fraction HF who developed CKD at the end of the 24-month follow up period (6.0% vs 5.8%, respectively) (Tables 4 and 5 of the supplementary data). In addition, in the overall study population, 6.4 per 100 patient-year developed type 2 diabetes during this period (Table 5), slightly more common in those patients with HF and reduced left ventricular ejection fraction than in those with HF and preserved left ventricular ejection fraction (Tables 4 and 5 of the supplementary data).
Percentage of patients who developed CKD within 24 months from HF diagnosis.
| 3 months | 6 months | 12 months | 24 months | Total | ||
|---|---|---|---|---|---|---|
| All HF patients who develop CKD according to CKD grade, n (%) | Stage 1 | 1 (20.0) | 3 (10.7) | 2 (6.3) | 4 (5.1) | 10 (7.0) |
| Stage 2 | 0 | 4 (14.3) | 4 (12.5) | 14 (17.9) | 22 (15.4) | |
| Stage 3a | 2 (40.0) | 8 (28.6) | 10 (31.3) | 22 (28.2) | 42 (29.4) | |
| Stage 3b | 2 (40.0) | 5 (17.9) | 8 (25.0) | 18 (23.1) | 33 (23.1) | |
| Stage 4 | 0 | 3 (10.7) | 5 (15.6) | 8 (10.3) | 16 (11.2) | |
| Stage 5 | 0 | 3 (10.7) | 0 | 4 (5.1) | 7 (4.9) | |
| Unspecified | 0 | 2 (7.1) | 3 (9.4) | 8 (10.3) | 13 (9.1) | |
| Total CKD | 5 (100) | 28 (100) | 32 (100) | 78 (100) | 143 (100) | |
| HF who stay without CKD, n (%) | Baseline | |||||
| All HF | 2435 | 2430 (99.8) | 2404 (98.7) | 2375 (97.5) | 2302 (94.5) | 2292 (94.1) |
| HF-rEF | 1223 | 1221 (99.8) | 1207 (87.7) | 1193 (97.5) | 1155 (94.4) | 1150 (94.0) |
| HF-pEF | 1212 | 1209 (99.8) | 1197 (98.7) | 1182 (97.5) | 1147 (94.6) | 1142 (94.2) |
CKD: chronic kidney disease; HF: heart failure; HF-rEF: heart failure with reduced ejection fraction; HF-pEF: heart failure with preserved ejection fraction.
Event rates per 100 patient-year for HF patients diagnosed in 2017 with or without CKD at baseline and followed for 24 months.
| HF without CKD at index (n=2435) | HF+CKD at index | Total HF (n=3045) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 (n=19) | Stage 2 (n=115) | Stage 3a (n=201) | Stage 3b (n=148) | Stage 4 (n=44) | Stage 5 (n=37) | Unspecified (n=46) | Total CKD (n=610) | PHFwithoutCKDvsHF+CKD | |||
| All-cause death, n (event rates) | 382 (16.1) | 2 (10.5) | 16 (14.9) | 35 (17.0) | 32 (21.3) | 11 (25.5) | 9 (27.6) | 6 (14.4) | 111 (21.9) | <.001 | 493 (17.2) |
| Time to first event, days | 426.9 | 446.4 | 429.9 | 396.8 | 330.7 | 264.6 | 231.5 | 438.2 | 348.1 | <.001 | 410.8 |
| HF, n (event rates) | 480 (20.2) | 2 (10.5) | 18 (16.2) | 37 (18.5) | 35 (23.1) | 12 (27.7) | 10 (30.0) | 7 (15.6) | 121 (23.8) | <.001 | 601 (20.6) |
| Time to first event, days | 393.9 | 499.8 | 481.3 | 444.2 | 370.2 | 296.2 | 259.1 | 490.5 | 366.5 | <.001 | 396.1 |
| CKD, n (event rates) | 214 (9.3) | 1 (5.3) | 8 (7.8) | 18 (9.0) | 17 (11.2) | 5 (13.4) | 5 (14.6) | 3 (7.6) | 57 (11.0) | .203 | 271 (9.4) |
| Time to first event, days | 441.2 | 626.5 | 603.3 | 556.9 | 464.0 | 371.2 | 324.8 | 614.9 | 450.5 | .359 | 447.5 |
| Albuminuria, n (event rates) | 238 (10.3) | 1 (5.3) | 9 (8.8) | 20 (10.1) | 20 (12.6) | 6 (15.1) | 5 (16.4) | 4 (8.5) | 65 (12.1) | <.001 | 303 (10.6) |
| Time to first event, days | 424.1 | 532.9 | 513.2 | 473.7 | 394.7 | 315.8 | 276.3 | 523.0 | 372.4 | <.001 | 421.8 |
| T2D development, n (event rates) | 149 (6.4) | 1 (5.3) | 6 (6.0) | 14 (6.9) | 13 (8.6) | 4 (10.3) | 4 (11.1) | 3 (5.8) | 45 (8.4) | <.001 | 194 (6.4) |
| Time to TD2 development, days | 413.1 | 515.9 | 496.8 | 458.6 | 382.1 | 305.7 | 267.5 | 506.3 | 376.7 | <.001 | 405.7 |
CKD: chronic kidney disease; HF: heart failure; T2D: type 2 diabetes.
Our study showed in a wide sample of patients representative of the Spanish population, that both prevalent and incident HF patients have many comorbidities and high risk of presenting cardiovascular and renal complications that could be partially related with an underuse of HF guidelines recommended therapies in a substantial proportion of patients.
At baseline, HF patients were old and had many comorbidities. Of note, the clinical profile of patients worsened with the presence of CKD. This is in line with previous studies performed in Spain and in other developed countries.21,22 In fact, our data were collected from the BIG-PAC database that comprised nearly 1.8 million persons daily attended in clinical practice and has been demonstrated its validity for observational studies and its representativeness of the Spanish population.19
On the other hand, approximately 52% of prevalent or incident patients had HF with reduced left ventricular ejection fraction and the remaining 48% preserved HF. First, it should be emphasized that in our study, in line with the DAPA-HF and DELIVER trials,14,20 preserved HF was defined as those patients with HF and a left ventricular ejection fraction>40%. However, current European guidelines define preserved HF with an ejection fraction≥50% and mildly reduced HF with an ejection fraction 41%–49%,1 and this could partially limit the interpretation of the data.
Previous studies have shown that HF with preserved ejection fraction accounts for at least half of the cases of HF, but reaches almost three-quarters of all HF patients among those subjects aged 65 years or older.17,18,23 Although previous studies have shown relevant differences in the clinical profile of patients with both entities, with more comorbidities in those patients with preserved HF,24,25 in our study baseline characteristics were quite similar. Although the definition of preserved HF was different in our study, these data suggest that the type of HF cannot be based solely on the presence of some particular conditions, but on an adequate diagnostic approach.1 In any case, although more evidences exist about treatments with reduced HF, overall, the management of HF patients is complex and requires a comprehensive approach.1
With regard to HF treatments, in the overall study population, around 30%–40% of patients were not taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers or sacubitril/valsartan, 30%–35% beta blockers, 70%–90% aldosterone antagonists and 95% SGLT-2 inhibitors (85% among patients with type 2 diabetes). In those patients with HF and reduced left ventricular ejection fraction, these numbers were 19%, 35%, 88% and 95% (83%), respectively. Although the prevalence of renal dysfunction was important, partially explained by the elderly population, some with hyperkalemia, and this could have had an impact on the management of these patients, overall, there was a substantial proportion of patients that were not taking those drugs that have demonstrated clinical benefits on reduced HF patients. It is likely that the use of oral potassium-binding agents in some cases could increase the use of renin–angiotensin system inhibitors.26 On the other hand, in the case of SGLT-2 inhibitors this low use could be explained because the DAPA-HF and the EMPEROR-Reduced trials were published in 2019 and 2020, respectively.14,15 However, the use of other HF guideline-directed medical therapies was too low, despite the clear recommendations performed by 2016 European guidelines, the current recommendations at the moment of the study.13 Although data provided from specific HF units show better numbers, studies performed in other clinical settings report similar figures than those observed in our study.27–30 As a result, more efforts are necessary to reduce the gap between guidelines recommendations and clinical practice.31,32 Of note, the 2021 European guidelines recommend the use of angiotensin-converting enzyme inhibitors/sacubitril-valsartan, beta blockers, aldosterone antagonists and SGLT-2 inhibitors as first line-therapy in the treatment of reduced HF patients,1 as this approach has been shown to significantly reduce mortality and HF hospitalization compared to standard HF therapy.33
Our study showed that the risk of outcomes in patients with HF remains unacceptably high. Thus, after only two years, rate of overall mortality was 17.2/100 patient-year and rates of hospitalization for HF and CKD were 20.6 and 9.4/100 patient-year. In a meta-analysis of 60 studies with data from 1.5 million people with HF, although the 5-year survival rates have improved in the last years (from 29.1% between 1970–1979 to 59.7% in 2000–2009), these remain very high.34 A recent study showed in patients with HF that 3 out of every 5 patients had died within 5 years of follow-up, with a median survival of 3 years.35 However, the elevated risk of outcomes in this population, particularly as renal function worsens, many patients do not receive evidence-based medical therapies, what worsens the prognosis.36 These data emphasize the need of prescribing recommended drugs as soon as possible to get the maximum benefit, as HF is a progressive condition without the appropriate treatment.37
Our study showed that among HF patients, renal function progressively decreased and albuminuria increased (11.5% and 7.2%, respectively) and 5.9% of patients developed CKD after only 2 years of follow-up. In addition, the risk of outcomes was higher in those patients with HF and CKD and time to first event was shorter (vs only HF alone) and worsened as CKD stage increased. Remarkably, although patients with HF and CKD were taking more angiotensin-converting enzyme inhibitors and angiotensin receptor blockers than those without CKD at baseline, the proportion of SGLT-2 inhibitors was similar. The relationship between HF and CKD is bidirectional and one condition promotes the development of the other and vice versa.38 Traditionally, renin–angiotensin system inhibitors have been the drugs used for the prevention and treatment of both, cardiovascular and renal complications, and their prescription should be promoted.1,39 Remarkably, in the last years, different clinical trials have shown that some SGLT-2 inhibitors can reduce the risk of cardiovascular and renal complications in patients with CKD (dapagliflozin in DAPA-CKD and canagliflozin in CREDENCE) and also in patients with HF and reduced left ventricular ejection fraction (dapagliflozin in DAPA-HF and empagliflozin in EMPEROR-Reduced).14,15,40,41 In addition, in patients with HF, SGLT2-inhibitors reduce the rate of kidney function decline, regardless baseline renal function.9,10 Unfortunately, our data showed that the current use of SGLT-2 inhibitors in this population is marginal. However, these drugs should be used to a higher extent, as their use is associated with a reduction of morbidity and mortality in patients with HF and reduced left ventricular ejection fraction, regardless of the renal function.42
Finally, although rates of outcomes were also high in patients with HF and preserved left ventricular ejection fraction, these seemed somewhat lower than in patients with reduced HF. This is in line with previous studies that have shown a similar or lower risk of mortality and cardiovascular complications in this population when compared to patients with reduced HF.23,24,43,44 Unfortunately, although some studies have suggested that some drugs could provide some benefits in patients with preserved HF,45,46 the fact is that the best approach in this population is a comprehensive management, treating adequately all comorbidities and congestive symptoms.1 However, at this moment different studies are being developed to assess the impact of SGLT-2 inhibitors on this population.20
LimitationsThis was an observational cohort study, with cross-sectional and longitudinal retrospective analyses that used secondary data from electronic health records. Therefore, only data that were recorded in the electronic clinical history could be collected, leading to a possible underdiagnosis of some comorbidities in some patients. In addition, data regarding the department where patients were managed (ie. HF unit, cardiology, internal medicine, intensive care unit, etc.) were not available in the database. However, although only indirect causality can be provided, this is the best design to actually represent clinical practice. In addition, the high number of patients included, as well as the robustness of the data, may reduce potential bias. On the other hand, no mildly reduced ejection fraction HF was considered as a separate entity in our study, as was included in the preserved HF cohort, what could limit the generalizability of the results.
In Spain, patients with HF are old, have many comorbidities and a high risk of developing cardiovascular and renal complications regardless of the ejection fraction group. Despite that, there is a substantial proportion of patients that are not taking guideline recommended drugs, partially due to the high prevalence of renal insufficiency. A higher use of these drugs could reduce HF burden in clinical practice.
- •
Current data about the management and cardiovascular and renal outcomes of patients with preserved and reduced HF are scarce.
- •
In Spain, patients with HF are old, have many comorbidities.
- •
HF patients have a high risk of developing cardiovascular and renal complications regardless of the ejection fraction group.
- •
Unfortunately, there is a substantial proportion of patients that are not taking guideline recommended drugs, partially due to the high prevalence of renal insufficiency.
This study was fully funded with an unrestricted grant of AstraZeneca.
Authors’ contributionsAll authors have contributed to the study design, result review, manuscript preparation and final approval of the manuscript.
Conflicts of interestNone.
Abbreviations: CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HF: heart failure; UACR: urine albumin-to-creatinine ratio; SGLT-2: sodium-glucose cotransporter-2.