Since the index cases were first reported in Wuhan, China, in December 2019, Coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) has become a global pandemic infecting more than 15.7 million individuals with more than 639 000 deaths worldwide by end of July 2020. Different molecules are being studied to prove their effectiveness as antivirals against the coronavirus (chloroquine, hydroxychloroquine, azithromycin, tocilizumab, remdesivir, lopinavir/ritonavir…).
Pending the results of ongoing randomized clinicals trials, the Moroccan Ministry of Health in concert with the Scientific Technical Committee of the Coronavirus Control Program decided to recommend the use of chloroquine or hydroxychloroquine in association with azithromycin as first-line treatment against COVID-19. However, both medications have been shown to increase the risk for QT-interval prolongation in other situations, with drug-induced torsades de pointes and drug-induced sudden cardiac death. Very few studies have focused on the evaluation of the safety profile of this combination therapy with high doses. The aim of the present investigation is to evaluate the safety of the abovementioned prescription.
This is a prospective observational study from Tangier University Hospital. All hospitalized patients with PCR-confirmed COVID-19 infection treated with chloroquine/hydroxychloroquine plus azithromycin were included from April 7 to May 6. PCR-confirmed COVID-19 patients were treated as follows: chloroquine (500mg twice a day for 10 days) or hydroxychloroquine (200mg 3 times a day, for 10 days) in combination with azithromycin (500mg on day 1, then 250mg a day from day 2 to 7). All data were collected, analyzed and verified by senior cardiologists. All patients had at least 3 electrocardiograms: on admission, on day 2, and at the end of treatment. Continuous monitoring by telemetry in high-risk patients (intensive care unit patients, medical history of arrhythmia or heart disease) was performed. Two physicians analyzed all electrocardiograms, with rhythm analysis, heart rate calculation, and systematic search for Q waves of necroses, atrioventricular block, right/left bundle branch, ventricular extrasystoles, torsade de pointes and ventricular arrhythmia; PR, QRS, QT and QT corrected intervals were measured manually. The lead showing the longest QT interval was used. The QTc is calculated according to the Bazett formula whenever the heart rate was between 50 and 100bpm and the rhythm was in regular sinus, and according to the FREDERICIA formula whenever the heart rate was below 50bpm or above 100bpm or the rhythm was irregular.
The prolongation was defined as QTc>460ms in women and >450ms in men, a severe prolongation was defined if QTc>500ms or QTc delta>60ms.1
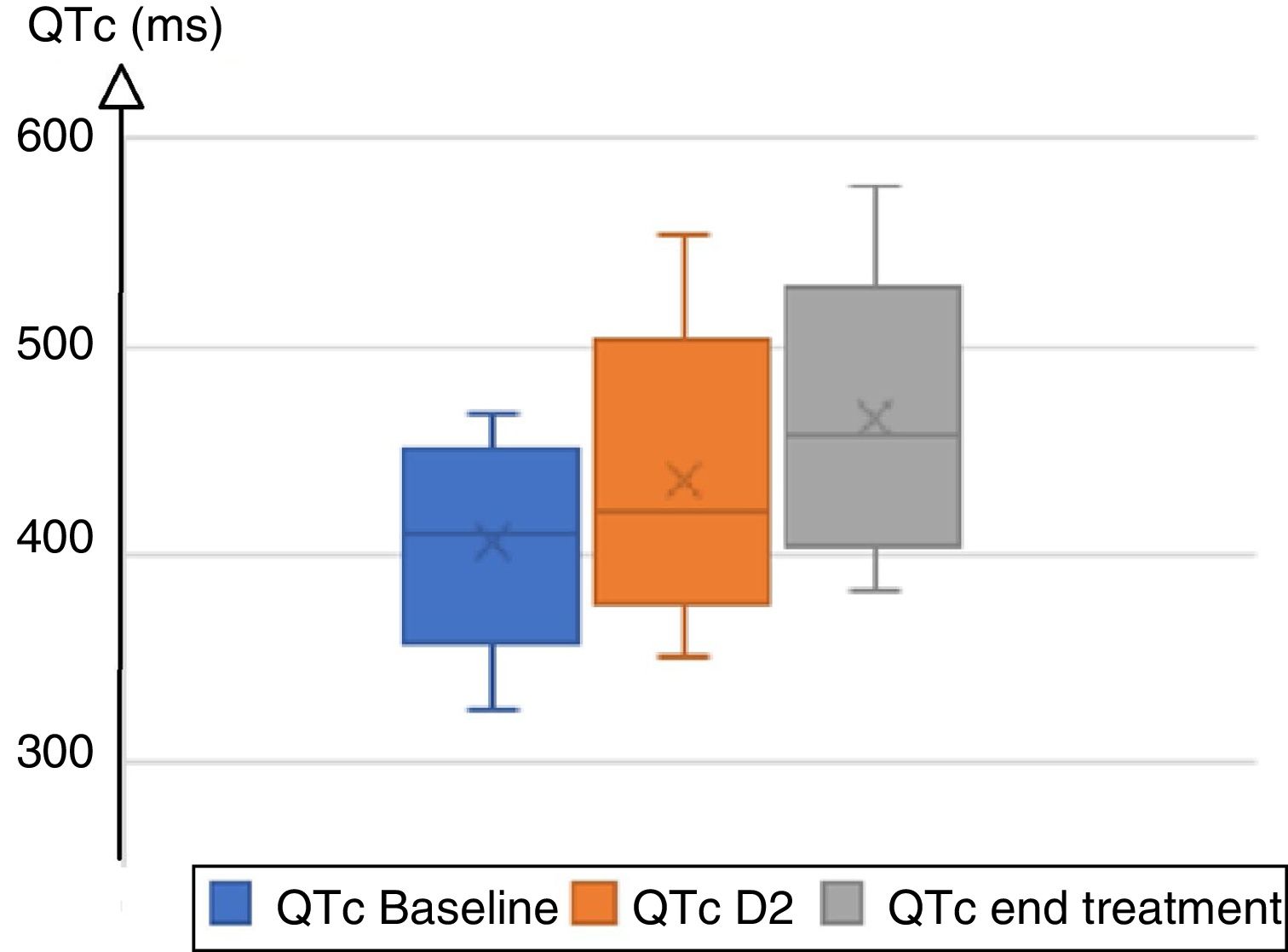
A total of 87 patients were treated with hydroxychloroquine/chloroquine plus azithromycin. Clinical and demographic characteristics are summarized in Table 1. On admission, 96.5% of patients were in sinus rhythm, 3 patients presented atrial fibrillation; the baseline heart rate was 83±15bpm. PR interval was 148±24ms; QRS duration was normal (88.6±12.6ms) with right bundle block in 15 individuals, and left bundle branch block in 2. The mean QTc duration at baseline was 410±31ms. During treatment, mean QRS increased from 88ms at baseline to 94ms on day 2, and to 97ms at the end of treatment, with a mean delta QRS (QRS max−initial QRS) at 11ms. Mean QTc increased from 410ms to 428ms on day 2, and 457ms at the end of treatment, with a mean delta QTc (QTc Max−initial QTc) at 31ms (Fig. 1). Four patients extended their QTc above 500ms. A total of 32 patients (36.7%) lengthened their QTc, including 20 women and 12 men (34.5% and 41.4% respectively). The chloroquine group lengthened QRS by 12.5±19.2ms and QTc by 35.9±43.8ms, while the hydroxychloroquine group lengthened QRS by 5.3±4.5ms and QTc by 15.3±33.8ms. Only 1 patient presented QTc prolongation in the hydroxychloroquine group vs 27 in the chloroquine group (9.0% vs 36.5%); 3 patients presented isolated asymptomatic ventricular extrasystoles, all in the chloroquine group. Patients that enlarged the QTc were older (48 vs 38 years old). The treatment protocol was interrupted in 2 patients due to major QTc prolongation beyond 550ms and in 3 intensive care patients for severe symptomatic bradycardia leading to atrioventricular block (grade II Mobitz 1 atrioventricular). Some monomorphic ventricular extrasystoles were noted, but no ventricular tachycardia or torsade de pointe was detected. A receiver operating characteristic (ROC) curve analysis was carried out to explore a QTc threshold at baseline for which there would be no risk of major QTc prolongation. There was no safety threshold for the initial QTc that allowed a safe prescription without ECG monitoring.
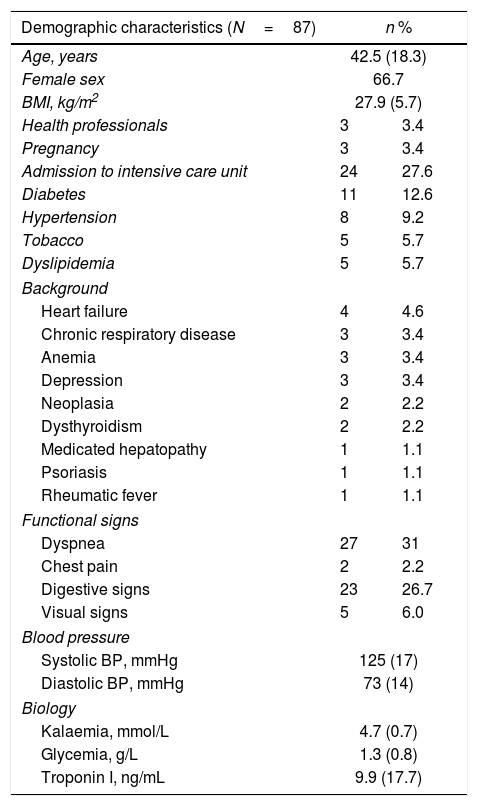
Clinical and demographic characteristics.
| Demographic characteristics (N=87) | n % | |
|---|---|---|
| Age, years | 42.5 (18.3) | |
| Female sex | 66.7 | |
| BMI, kg/m2 | 27.9 (5.7) | |
| Health professionals | 3 | 3.4 |
| Pregnancy | 3 | 3.4 |
| Admission to intensive care unit | 24 | 27.6 |
| Diabetes | 11 | 12.6 |
| Hypertension | 8 | 9.2 |
| Tobacco | 5 | 5.7 |
| Dyslipidemia | 5 | 5.7 |
| Background | ||
| Heart failure | 4 | 4.6 |
| Chronic respiratory disease | 3 | 3.4 |
| Anemia | 3 | 3.4 |
| Depression | 3 | 3.4 |
| Neoplasia | 2 | 2.2 |
| Dysthyroidism | 2 | 2.2 |
| Medicated hepatopathy | 1 | 1.1 |
| Psoriasis | 1 | 1.1 |
| Rheumatic fever | 1 | 1.1 |
| Functional signs | ||
| Dyspnea | 27 | 31 |
| Chest pain | 2 | 2.2 |
| Digestive signs | 23 | 26.7 |
| Visual signs | 5 | 6.0 |
| Blood pressure | ||
| Systolic BP, mmHg | 125 (17) | |
| Diastolic BP, mmHg | 73 (14) | |
| Biology | ||
| Kalaemia, mmol/L | 4.7 (0.7) | |
| Glycemia, g/L | 1.3 (0.8) | |
| Troponin I, ng/mL | 9.9 (17.7) | |
Data are expressed as no. (%) or mean (±standard deviation).
BMI, body mass index; BP, blood pressure.
Chloroquine/hydroxychloroquine are related to type 1a antiarrhythmics and act as membrane stabilizers and cause QRS and QT prolongation.2 The association induced mild QTc prolongation when given to young volunteers in good health.
In our study, the association of chloroquine/hydroxychloroquine and azithromycin induced significant QTc prolongation in 33 of 87 patients (38%), but a severe prolongation was noted in only 13 (15%) patients (QTc>500ms or delta QTc>60ms). A significant QRS enlargement was noted, but no pathological prolongation was found (QRS>120ms or delta QRS>30%). Besides, no arrhythmic deaths have been reported in our series.
We found that the chloroquine group markedly increased QTc and QRS duration compared to the hydroxychloroquine group. Hydroxychloroquine seems to be safer.
Advanced age was the only significant factor in the QTc prolongation. The severe prolongation leading to treatment interruption was found in only 5 patients (6%). There were no cases of torsade de pointes or arrhythmic death in the entire cohort. Our results are in line with the literature. In the cohort of Saleh et al., 119 (59.2%) patients received the combination therapy, a peak Qtc>500ms was observed in 11 (9.2%) (P=1.00) without cases of torsade de pointes or arrhythmic death.3 In their study of 84 patients receiving the regimen, Chorin et al. showed that the QTc was severely prolonged to > 500ms in 11% of patients. No torsade de pointes events recorded.4
Interestingly, in their open-label non-randomized clinical trial of 1061 SARS-CoV-2 positive patients treated by the association, Raoult et al. report that only 9 had a QTc prolongation of more than 60ms from baseline but no one exceeded 500ms. No arrhythmogenic events or deaths were reported.5 Mercuro et al. reported one case of torsades de pointes among the 11 patients with prolonged QTc.1 We observed that in our cohort there was no greater prolongation of the QT interval in women, and that age was the only factor implicated in this prolongation (> 48 years old).
Unfortunately, our study does not allow to suggest a safety threshold of the initial QTc for which there would be no major prolongation of the QTc, but we still believe that we can potentially suggest an early discharge with ambulatory follow-up for some selected patients, such as those younger, without cardiovascular risk factors or any other medication known to be associated with QTc prolongation.
An innovative approach has been described in a recent publication, proposing mobile cardiac outpatient telemetry for QTc and arrhythmia monitoring, and minimizing both health professionals’ exposure and personal protective equipment use.6 Moreover, this tool is not easily available in the majority of healthcare systems; other alternatives such as programs on smartwatches are currently under development for a better care optimizing. Our study has some limitations, a low sample size and a relatively young population; the results should be taken with caution and cannot be extrapolated.
The association hydroxychloroquine/chloroquine and azithromycin for the treatment of COVID-19 was associated with QTc prolongation in one third of patients, but no major events were noted.