Paroxysmal and asymptomatic episodes of atrial fibrillation (AF) can be the cause of previously diagnosed cryptogenic stroke. Guidelines recommend monitoring in this setting, although the optimum duration and method remains unknown. We aimed to determine the diagnostic yield of a sequential monitoring approach using external and internal loop recorders for detecting AF in patients with cryptogenic stroke after a complete diagnostic evaluation.
MethodsA total of 119 patients were enrolled. A 24-h Holter was performed in all patients. An ambulatory external loop recorder was scheduled in patients without evidence of AF on Holter, followed by ambulatory internal loop recorder implantation in cases without evidence of AF at this point.
ResultsAF was identified in 43 of 119 patients (36%). The rate of detection was 15.1% at 6 months, 24.3% at 12 months, 35.3% at 24 months, and 36.1% at 36 months. AF was detected in 21/112 patients (18.75%) with the external loop recorder, and in 17/80 (21%) with internal loop recorder.
ConclusionsA standardized sequential monitoring strategy in patients in whom an embolic source is suspected detects AF frequently, allowing a reduction in insertable loop recorder implantation as in 60% of cases AF is detected using non-invasive methods.
La fibrilación auricular (FA), de naturaleza frecuentemente paroxística y asintomática, puede ser causa de ictus criptogénico. Las actuales guías de manejo clínico recomiendan la monitorización de estos pacientes, aunque el método y la duración óptimos de la misma permanecen controvertidos. Nuestro objetivo fue determinar el rendimiento diagnóstico de una aproximación secuencial en esta monitorización, utilizando inicialmente registradores externos, e implantables en un escalón posterior, para la detección de FA en pacientes con ictus criptogénico tras una evaluación completa.
MétodosSe incluyeron 119 pacientes. En todos ellos se realizó monitorización Holter convencional de 24h. En pacientes sin FA en la monitorización se colocó un registrador externo, seguido de uno implantable en aquellos casos sin evidencia de ritmos embolígenos tras el mismo.
ResultadosSe identificó FA en 43 de los 119 pacientes (36%). La tasa de detección fue de un 15,1% a los 6 meses, un 24,3% a los 12 meses, un 35,3% a los 24 meses y un 36,1% a los 36 meses. Se detectó FA en 21/112 (18,75%) pacientes portadores del registrador externo, y en 17/80 (21%) de aquellos en los que se llegó a colocar el dispositivo implantable.
ConclusionesUna estrategia secuencial y estandarizada de monitorización de ritmo cardiaco en pacientes con ictus criptogénico en los que se sospecha una fuente embólica detecta frecuentemente FA y permite reducir la tasa de implante de dispositivos insertables, dado que un 60% de los casos se diagnosticó con métodos no invasivos.
Cardioembolism accounts for 17–35% of all ischemic strokes. In comparison to other stroke etiologies it carries a higher risk of death and recurrent stroke, and a higher risk of severe disability. However, it is estimated that up to 30% of ischemic strokes, labeled cryptogenic ischemic strokes, have an undetermined cause.1–4 Early diagnosis of cardioembolic sources is important in order to initiate anticoagulant therapy, shown to be much more effective than antiplatelet agents for preventing cardioembolic stroke.5 Improved efforts to detect atrial fibrillation (AF) in this subgroup are warranted, as AF is a high-risk source of cardioembolism. Furthermore, detection of AF after cryptogenic ischemic stroke further increased the risk of recurrent stroke, even when compared to patients with known AF.6
Detection of AF in this setting can be challenging given the paroxysmal and asymptomatic nature of arrhythmia episodes.7 Although screening of AF has been traditionally limited to short-duration electrocardiogram (ECG) post-stroke monitoring (mainly 24-h Holter), longer duration significantly improves detection rates, and it is recommended in recent guidelines. However, due to the limitations of existing studies, guidelines have yet to endorse specific strategies for detecting AF in patients with cryptogenic ischemic stroke. The optimum monitoring duration and method of AF detection after stroke remain unknown.8–10
We aim to determine the yield of AF detection in patients with cryptogenic ischemic stroke after a complete diagnostic evaluation, based on the sequential use of diagnostic methods.
MethodsEligibility criteriaAll patients were older than 18 years of age and had received a diagnosis of ischemic stroke or transient ischemic attack (TIA) of undetermined cause according to TOAST (Trial of Org 10172 in Acute Stroke Treatment criteria).11 Stroke was classified as cryptogenic by a stroke neurologist after extensive testing, that included 12-lead ECG, at least 72h of in-hospital ECG monitoring, cardiac echocardiography (including transesophageal study in patients <55 years-old, or to rule out patent foramen ovale after bubble testing), screening for hypercoagulable states (in patients <55 years of age), and arterial imaging studies to asses cervical and intracranial arteries and aortic arch (cervical and intracranial ultrasonography and/or computed tomography angiography and/or magnetic resonance angiography (MRA) and/or arteriography).
Patients with TIA were included only if symptoms at presentation were consistent with an embolic event, such as speech problems, limb weakness or hemianopsia.
Stroke severity was rated using the National Institutes of Health (NIH) Stroke Scale.12 Stroke severity and location were also assessed by the Oxfordshire Community Stroke Project classification.13 The degree of dependence at 3 months using the modified Rankin scale and the presence of old infarcts in the neuroimaging tests were also collected.14
The main exclusion criteria were the previous history of AF or flutter. Patients with an indication for a pacemaker or implantable cardioverter-defibrillator were also excluded. Therefore, patients were excluded if the most likely etiologic diagnosis has already been determined (large-vessel or small-vessel disease, for instance).
Baseline assessmentAll data regarding patient's medical history, physical examination findings, laboratory testing and use of medications, were prospectively recorded in a dedicated database. Information regarding the index stroke or TIA was collected, including the results of brain imaging and the required tests to establish a consistent diagnosis of cryptogenic ischemic stroke.
Monitoring strategiesAll patients had a standard 12-lead ECG at admission, followed by continuous monitoring for at least 72h in the stroke unit at University Hospital Marques de Valdecilla, Santander, Spain. In the absence of AF on this initial period, patients were included in a sequential protocol for the detection of potentially embolic rhythms. All patients provided written informed consent. A 24-h 3-channel ECG recording (Holter) was performed in all patients. An ambulatory external loop recorder (ELR) (Spider Flash, Sorin-Livanova group) was scheduled in patients without evidence of AF on Holter. Patients were instructed to wear the monitor as much as possible for 14 days. An ambulatory internal loop recorder (ILR) (Reveal XT, Medtronic) was scheduled in patients without evidence of AF on ELR. Both ELR and ILR automatically detect and record AF via R–R variability pattern recognition, irrespective of heart rate or symptoms.
All the episodes of AF were adjudicated by a cardiac electrophysiologist. AF was defined as an episode of irregular rhythm without detectable P waves, lasting more than 30″.
Patients underwent an assessment at scheduled visits at 1 month, and every 3 months thereafter, with unscheduled visits in the event of symptom occurrence. Decisions regarding anticoagulant therapy were made at the discretion of the treating physicians.
Statistical analysisSPSS version 15.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. A P value <.05 was accepted as statistically significant. Continuous data are presented as mean±standard deviation or median. Comparisons between groups were completed with Student t test or the Mann–Whitney U test. Categorical variables were assessed with chi-square test. For survival analysis, Kaplan–Meier survival analysis was performed.
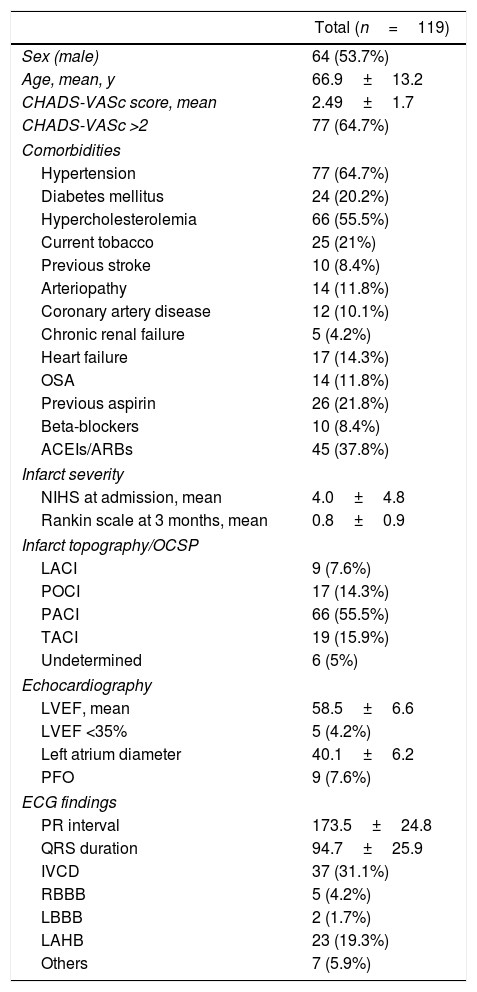
ResultsPatient populationFrom January 2014 to January 2016, a total of 119 patients were enrolled. Baseline characteristics and infarct topography are shown in Table 1. Mean age was 66.9±13.2 years, and 53.7% of patients were male (n=64). The index event was ischemic stroke in 84 patients (70.6%) and a TIA in 35 (29.4%).
Patient's characteristics and comorbidities.
| Total (n=119) | |
|---|---|
| Sex (male) | 64 (53.7%) |
| Age, mean, y | 66.9±13.2 |
| CHADS-VASc score, mean | 2.49±1.7 |
| CHADS-VASc >2 | 77 (64.7%) |
| Comorbidities | |
| Hypertension | 77 (64.7%) |
| Diabetes mellitus | 24 (20.2%) |
| Hypercholesterolemia | 66 (55.5%) |
| Current tobacco | 25 (21%) |
| Previous stroke | 10 (8.4%) |
| Arteriopathy | 14 (11.8%) |
| Coronary artery disease | 12 (10.1%) |
| Chronic renal failure | 5 (4.2%) |
| Heart failure | 17 (14.3%) |
| OSA | 14 (11.8%) |
| Previous aspirin | 26 (21.8%) |
| Beta-blockers | 10 (8.4%) |
| ACEIs/ARBs | 45 (37.8%) |
| Infarct severity | |
| NIHS at admission, mean | 4.0±4.8 |
| Rankin scale at 3 months, mean | 0.8±0.9 |
| Infarct topography/OCSP | |
| LACI | 9 (7.6%) |
| POCI | 17 (14.3%) |
| PACI | 66 (55.5%) |
| TACI | 19 (15.9%) |
| Undetermined | 6 (5%) |
| Echocardiography | |
| LVEF, mean | 58.5±6.6 |
| LVEF <35% | 5 (4.2%) |
| Left atrium diameter | 40.1±6.2 |
| PFO | 9 (7.6%) |
| ECG findings | |
| PR interval | 173.5±24.8 |
| QRS duration | 94.7±25.9 |
| IVCD | 37 (31.1%) |
| RBBB | 5 (4.2%) |
| LBBB | 2 (1.7%) |
| LAHB | 23 (19.3%) |
| Others | 7 (5.9%) |
ACEIs, angiotensin-converting enzyme inhibitor; ARBs, angiotensin receptor blocker; ECG, electrocardiogram; IVCD, intraventricular conduction defect; LACI, lacunar infarction; LAHB, left anterior hemiblock; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NIHS, National Institutes of Health Stroke Scale; OCSP, Oxfordshire Community Stroke Project classification; OSA, obstructive sleep apnea; PACI, partial anterior circulation infarction; POCI, posterior circulation infarction; PFO, permanent foramen ovale; RBBB, right bundle branch block; TACI, total anterior circulation infarction.
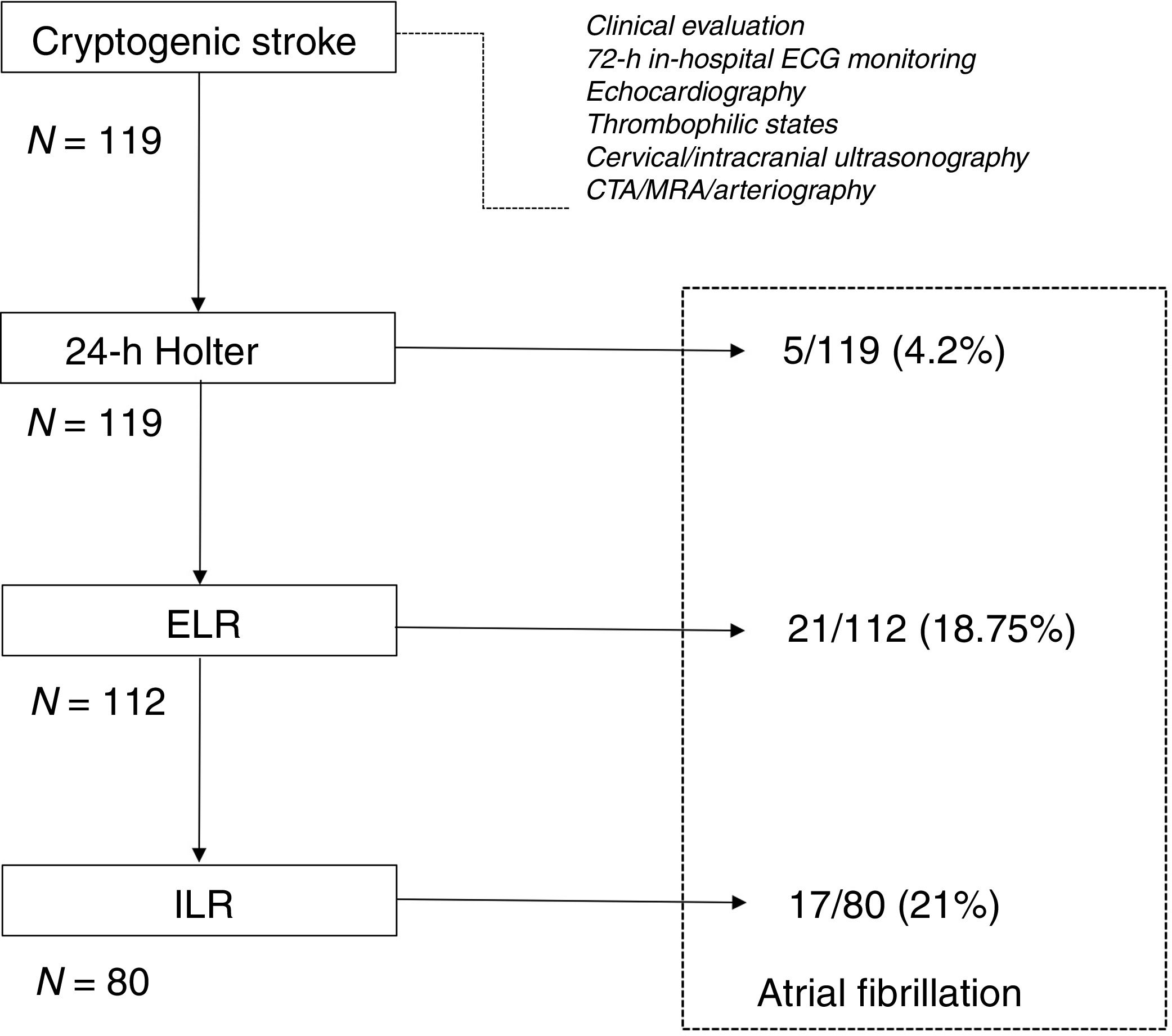
AF was detected in 43 of 119 patients (36%, Fig. 1). The rate of detection was 15.1% at 6 months, 24.3% at 12 months, 35.3% at 24 months, and 36.1% at 36 months.
After the initial 24-h Holter monitoring (during the first week after the index event), 5 (4.2%) of the 119 patients had AF diagnosed.
A total of 112 undiagnosed patients underwent ELR monitoring and received the device a mean time of 26.5±12.3 days after the index event. Mean monitoring period was 10.4±1.9 days. AF was detected in 21/112 patients (18.75%) after a mean period of 5.7 days (median 4 days). In 64.7% of patients, the AF was discovered within the first 7 days.
Of the 91 patients whose ELR was nondiagnostic, 80 underwent ILR implantation after a mean time of 47.1±18.2 days after the index event, 8 patients refused this invasive procedure, 1 moved to another city and was lost to follow-up, and 2 other patients were diagnosed with a systemic disease (vasculopathy and solid organ carcinoma) and exited the study.
The rate of detection of AF with the ILR was 21% (17/80). AF was asymptomatic in all patients but one. The maximum 1-day duration of AF was more than 12h in 37.5% of patients, more than 4–12h in 31.3%, more than 1–4h in 12.5% of patients, and 1h or less in 15% of patients. The mean time to detection of AF with the ILR was 7.9±7 months (median 5). In 13 patients (16.2%) automatic AF episodes detected by the device were classified as false positive events, mainly due to atrial and/or ventricular premature complexes.
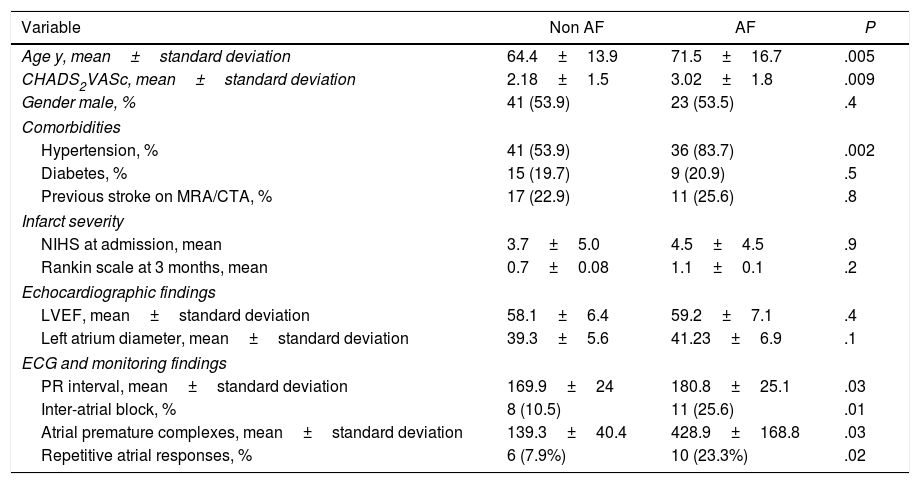
Differences between groupsThe characteristics of the groups with and without AF are reflected in Table 2.
Univariate analysis of risk factors for AF diagnosis after cryptogenic stroke.
| Variable | Non AF | AF | P |
|---|---|---|---|
| Age y, mean±standard deviation | 64.4±13.9 | 71.5±16.7 | .005 |
| CHADS2VASc, mean±standard deviation | 2.18±1.5 | 3.02±1.8 | .009 |
| Gender male, % | 41 (53.9) | 23 (53.5) | .4 |
| Comorbidities | |||
| Hypertension, % | 41 (53.9) | 36 (83.7) | .002 |
| Diabetes, % | 15 (19.7) | 9 (20.9) | .5 |
| Previous stroke on MRA/CTA, % | 17 (22.9) | 11 (25.6) | .8 |
| Infarct severity | |||
| NIHS at admission, mean | 3.7±5.0 | 4.5±4.5 | .9 |
| Rankin scale at 3 months, mean | 0.7±0.08 | 1.1±0.1 | .2 |
| Echocardiographic findings | |||
| LVEF, mean±standard deviation | 58.1±6.4 | 59.2±7.1 | .4 |
| Left atrium diameter, mean±standard deviation | 39.3±5.6 | 41.23±6.9 | .1 |
| ECG and monitoring findings | |||
| PR interval, mean±standard deviation | 169.9±24 | 180.8±25.1 | .03 |
| Inter-atrial block, % | 8 (10.5) | 11 (25.6) | .01 |
| Atrial premature complexes, mean±standard deviation | 139.3±40.4 | 428.9±168.8 | .03 |
| Repetitive atrial responses, % | 6 (7.9%) | 10 (23.3%) | .02 |
CTA, computed tomography angiography; ECG, electrocardiogram; LVEF, left ventricular ejection fraction; MRA, magnetic resonance angiography; NIHS, National Institutes of Health Stroke Scale.
Patients with AF detected were significantly older, with higher values in CHADS2VASc scale, and greater prevalence of hypertension. In baseline ECG, the interatrial block was more prevalent in those with AF identified. These patients also had more prolonged PR interval and exhibited more frequent atrial premature complexes and repetitive nonsustained atrial tachycardia in 24-h Holter monitoring.
We do not find any pattern of acute brain infarction that was significantly associated with AF detection in the follow-up, neither was the presence of chronic brain infarctions on magnetic resonance angiography/computed tomography angiography.
Medical managementAt hospital discharge, the majority of patients were receiving antiplatelet therapy (104/119, 87.4%), and a minority (15/119) started empirical oral anticoagulation (mainly because of thrombus extraction during catheter-based procedures, or when recurrences). After monitoring, patients with AF were changed to direct oral anticoagulants, although 3 patients already on oral anticoagulation with excellent times on therapeutic range continued on acenocumarol.
One patient with idiopathic cardiomyopathy and severe left ventricular dysfunction experienced a syncopal event, with ventricular tachycardia diagnosed on ILR monitoring, and underwent ICD implantation.
SafetyOf 80 ILR that were inserted, 1 (1.25%) was removed owing to infection at the insertion site. No patients had a recurrent stroke or TIA during the study period.
DiscussionThe main findings of this study can be summarized as follows: (a) a standardized sequential monitoring strategy is feasible to implement as part of routine stroke care in patients in whom an embolic source is suspected, and detected AF in 35% of patients; (b) in 60% of cases, AF is detected using noninvasive methods, mainly ELR; (c) this approach may allow a reduction in ILR implantation without a significative impairment in diagnostic yield.
Results of previous studies indicate that prolonged monitoring of heart rhythm should become part of the standard care of patients with cryptogenic ischemic stroke. A growing body of evidence indicates that AF likely accounts for a proportion of these events.15–23 Guidelines recommend that the evaluation of patients who present with ischemic stroke should include monitoring for AF, although duration varies across different guidelines.8–10 However, owing to the limitations of existing studies, guidelines have yet to endorse specific strategies for detecting AF, and the optimum monitoring method of AF detection after stroke remains unknown.24 Even more, in a routine basis persists an evidence-practice gap with underutilization of ECG monitoring in these patients.25 Detection of AF has therapeutic implications because documentation of AF is required to initiate anticoagulant therapy after ischemic stroke, while antiplatelet agents are recommended in the absence of AF.
The main available technologies for arrhythmia detection in this setting were validated in the EMBRACE (30-day cardiac Event Monitor Belt for Recording Atrial fibrillation after a Cerebral ischemic Event) trial, using ELR, and the CRYSTAL-AF (CRYptogenic Stroke and underlying Atrial Fibrillation) trial, using an ILR.15,26 As the yield of AF detection continues to increase with increasing duration of the monitoring time, ILRs represent the most sensitive method for detecting sporadic episodes of AF. However, they are costly, (minimally) invasive and not yet widely accessible. Relatively inexpensive ELR, such as Spider Flash reported herein, will probably be cost-effective, although patient compliance may decline rapidly with increased monitoring durations.
We performed 24-h Holter ECG as initial screening in an out-patient setting. Our detection rate of 4% is in consonance with published rates of 2–6%.27–29 When AF has not detected patients underwent additional noninvasive ECG monitoring with Spider-Flash for 2 weeks. This duration of monitoring may be reasonable given the fact that most cases in the EMBRACE trial were detected within the first 2 weeks of monitoring and may be associated with better patient compliance than the 30 days reflected in guidelines without significantly affecting detection rate. In fact, our detection rate of 18% at this point is slightly higher than the one reported in the EMBRACE trial. Time delay after index event may be relevant for diagnostic yield with the ELR, as AF recurrences are clustered. Those patients without AF at this point underwent ILR implantation, with a detection rate of 20%, close to previous noncontrolled trials.
This sequential strategy resulted in a high rate of detection of AF, similar to detection rates reported in studies using directly ILR.23,30 This result should be viewed in the context of the use of a comprehensive systematic baseline diagnostic evaluation to rule out other causes of stroke, including imaging modalities not used in the EMBRACE, in consonance with the diagnostic criteria and assessment recently recommended for the definition of the embolic stroke of unknown etiology.31 Besides, our results may be explained in part because of differences in baseline characteristics, including age, the prevalence of hypertension and severity of stroke (NIHS). The incidence of AF goes up with age and hypertension, and large and severe strokes are likely to be more cardioembolic in origin. All of these factors are increased in our patients compared to patients enrolled in EMBRACE and CRYSTAL trials, for instance, and may explain much of the differences between studies.
Further studies with larger number of patients are needed to determine which risk factors identify the patient who would derive the most clinical benefit for this prolonged monitoring. CHADS2 and CHA2DS2-VASc scores, systematically higher in those patients with AF detected after cryptogenic ischemic stroke have been shown to predict embolic events in patients other than those with established AF.32 Usefulness of these scores as a tool for identifying patients to propose for more prolonged rhythm monitoring merits further exploration. In our series, we found that the number of Holter detected atrial premature beats, runs of nonsustained atrial tachycardia, long PR interval or the presence of interatrial block in the surface ECG were associated with detection of AF. This is in consonance with previous reports,33–35 and suggests that these patients may have a high probability of AF in the context of an atrial myopathy, and could represent ideal candidates for prolonged ECG monitoring. In our patients, we do not find any pattern of acute brain infarction that was significantly associated with AF.36
We propose a sequential approach for detecting AF, starting with noninvasive ELR that may avoid a significant number of ILR. Although a similar approach has been recently suggested by Gladstone et al.,37 to the best of our knowledge it has not been prospectively evaluated. In our series, this monitoring strategy resulted in a high rate of detection of AF, with subsequent oral anticoagulation therapy initiation.
LimitationsThe main limitation of our study is the relatively low number of patients included, although it is similar to previous nonrandomized studies of ELR/ILR. On the other hand, one of the strengths of our study is the use of a comprehensive systematic baseline diagnostic evaluation to rule out other causes of stroke.
ConclusionsA sequential approach to AF detection in patients with embolic stroke of unknown source after complete diagnostic assessment identifies subclinical AF in 35% of cases. We suggest a 24-h Holter ECG as an initial screen. If AF is not detected additional non-invasive ECG monitoring with ELR for 2 weeks, followed by ILR is performed. This strategy allows the diagnosis with noninvasive methods in more than half of the patients with definitive AF. AF diagnosis provided a firm indication for a switch from antiplatelet therapy to therapeutic anticoagulation. Further studies are needed to determine which risk factors identify patients who would obtain the greatest clinical benefit.
- -
Current guidelines recommend that the evaluation of patients who present with ischemic stroke of unknown etiology should include monitoring for AF, as a growing body of evidence suggests that AF likely accounts for a proportion of these events.
- -
However, the duration of this monitoring period varies across different guidelines, and the optimum monitoring method of AF detection after stroke remains unknown.
- -
We propose a sequential approach for detecting AF as part of routine stroke care in patients in whom an embolic source is suspected, starting with noninvasive ELR that may avoid a significant number of ILR without a significative impairment in diagnostic yield. This monitoring strategy resulted in a high rate of detection of AF (35% of patients). AF diagnosis provided a firm indication for a switch from antiplatelet therapy to therapeutic anticoagulation.
None.
Abbreviations: AF, atrial fibrillation; ELR, external loop recorder; TIA, transient ischemic attack.