Evaluate patients referred for cardiac rehabilitation to determine the prevalence of cognitive impairment (CI) and compare readmission rates and mortality for those with and without CI.
MethodsPatients were retrospectively divided into cohort A (Mini-Cog completed) and cohort B (Mini-Cog not completed). Cohort A was then divided into A1 (Mini-Cog positive for CI) and A2 (Mini-Cog negative for CI).
ResultsOf 1440 patients, 986 (68%) completed the Mini-Cog (cohort A) and 454 (32%) patients did not (cohort B). Within cohort A, 46 (4.7%) had a positive Mini-Cog (cohort A1) and 940 (95.3%) had a negative Mini-Cog (cohort A2). Cohort A1 had significantly higher rates of all-cause readmission compared with cohorts A2 and B (63% vs 44% and 47%; P=.02), and significantly higher mortality (28% vs 9% vs 15%; P<.001), but was also significantly older, with more co-morbidities. After accounting for demographic and co-morbidity differences between cohorts A1 and A2 using propensity score matching and Cox proportional hazards model, cohort A1 had significantly increased rates of the composite outcome of readmission and/or death at 3-months (P=.002).
ConclusionsPoor performance on the Mini-Cog identified an older group of phase I cardiac rehabilitation patients that had significantly increased rates of the combined end-point of readmission plus death.
Evaluar pacientes derivados a rehabilitación cardiaca para determinar la prevalencia de deterioro cognitivo (DC) y comparar tasas de reingreso y mortalidad entre aquellos con y sin DC.
MétodosSe dividió a los pacientes retrospectivamente en cohorte A (Mini-Cog completado) y cohorte B (Mini-Cog no completado). La cohorte A se dividió a su vez en A1 (Mini-Cog positivo para DC) y A2 (Mini-Cog negativo para DC).
ResultadosDe 1.440 pacientes, 986 (68%) completaron el Mini-Cog (cohorte A) y 454 (32%) pacientes no lo hicieron (Cohorte B). Dentro de la cohorte A, 46 (4,7%) tenían un Mini-Cog positive para DC (cohorte A1) y 940 (95,3%) tenían un Mini-Cog negativo (cohorte A2). La cohorte A1 tuvo tasas significativamente más altas de reingreso por cualquier causa en comparación con las cohortes A2 y B (63 frente a 44% y 47%; p = 0,02) y una mortalidad significativamente mayor (28 frente a 9 frente a 15%; p < 0,001), pero los pacientes también eran significativamente más mayores y con más comorbilidades. Después de tener en cuenta las diferencias demográficas y la comorbilidad entre las cohortes A1 y A2 mediante el emparejamiento por puntuación de propensión y el modelo de riesgos proporcionales de Cox, la cohorte A1 aumentó significativamente las tasas de reingreso o muerte a los 3 meses (p = 0,002).
ConclusionesUn resultado deficiente en el Mini-Cog identificó a un grupo de pacientes de rehabilitación cardiaca en fase I más ancianos con tasas de eventos combinados de reingresos y muerte significativamente mayores.
Mild cognitive impairment is a pre-cursor to dementia and Alzheimer's disease.1 Cognitive impairment is associated with increased rates of readmission, longer hospitalizations, and increased mortality in elderly patients.2–4
The Mini-Cog test is a quick and easy test that has been shown to have good accuracy at identifying cognitive impairment.4,5 Several cardiac populations with cognitive impairment, identified on Mini-Cog, have poor post-hospital discharge outcomes.6–10 While cognitive impairment is shown to be fairly common in heart failure patients, it is less established what the prevalence is in a broader cardiology cohort, and how significantly it impacts mortality and readmission rates in this broader population.8–12
Since April of 2015 the cardiopulmonary rehabilitation therapists at our institution have administered the Mini-Cog test to patients referred for inpatient (phase I) cardiopulmonary rehabilitation. Our study aims were to evaluate a large, consecutive cohort of patients referred for cardiopulmonary rehabilitation and determine the prevalence of cognitive impairment and compare readmission rates and mortality for those with and without cognitive impairment. We hypothesized that hospitalized patients receiving cardiopulmonary rehabilitation that perform poorly on the Mini-Cog would identify a group at high-risk for readmission and death.
MethodsThe Mini-Cog exam was prospectively ordered on 1627 consecutive cardiopulmonary rehabilitation patient encounters from 1 March 2016 to 28 February 2017 (Fig. 1). Each patient's electronic medical record (EMR, Epic, United States) was retrospectively reviewed to collect and record study data. The institutional review board approved the study with a waiver of patient consent. Seven patients were excluded from the study cohort, as they died during their index admission. There were 152 patients that had more than one phase I cardiopulmonary rehabilitation order during the study period. Only the initial Mini-Cog score and associated data were included to remove duplicate patients from contributing to the outcome data.
Mini-Cog examThe cardiopulmonary rehabilitation therapist administered the exam to patients using the following protocol:
- •
Instruct the patient to listen carefully and remember the following three words: table, car, orange.
- •
The patient was then provided with a paper that had a blank circle and instructed to draw the face of a clock.
- •
Once the clock face was drawn then the patient was instructed to position the hands of the clock to a time of 9:10.
- •
The patient was then asked to recite the three memory words.
- •
One-point for each word correctly recalled, for a possible total of three points.
- •
One-point for correctly drawing the clock face.
- •
One-point for correctly positioning the clock hands to demonstrate a time of 9:10.
- •
A score of 2 or less was considered a positive Mini-Cog screen for cognitive impairment. The patient's registered nurse was notified of abnormal test results.
- •
A score of 3 or higher was considered negative for cognitive impairment.
Once the final cohort was created patients were then divided into cohort A (Mini-Cog completed) and cohort B (Mini-Cog not completed). Cohort A was then further divided into A1 (Mini-Cog positive for cognitive impairment, with a score of 2 or less) and A2 (Mini-Cog negative for cognitive impairment, with a score of 3 or better). Patients were followed in their EMR through a censure date of 31 October 2017 to evaluate for readmissions to HealthPartners Hospitals (HealthPartners is an integrated healthcare system based in Minnesota and Western Wisconsin that includes 8 hospitals). Care Everywhere was used to identify hospitalizations that occurred outside the HealthPartners system. The Minnesota Department of Health's Death Search was utilized to determine any deaths occurring prior to our censure date – 31 October 2017. To assess for death in Western Wisconsin residents lost to follow-up, we evaluated obituaries in local newspapers.
StatisticsContinuous data are presented as mean±one standard deviation. Categorical data are presented as numbers, with percentages provided in parenthesis. Comparisons were performed using a t-test, contingency table, analysis of variance or Chi-square. A P-value≤.05 was considered statistically significant.
Age, gender, and co-morbidities differed between groups (A1 and A2). Therefore, we used propensity score matching to mimic a randomized clinical trial to control for the impact of these potential confounders on the treatment assignment (A1 and A2) and the outcome. We matched on age, gender and 9 different co-morbidities: hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, chronic kidney disease, chronic obstructive pulmonary disease, congestive heart failure, cerebrovascular accident, and current smoking. We first attempted 3 main methods of matching: nearest neighbor matching, optimal full matching, and exact matching. Nearest neighbor matching method was also tested on 5 different specifications: greedy nearest neighbor with logistic regression for propensity score, nearest neighbor with caliper, with ratio k:1 matching, matching with probit regression for propensity score, and nearest neighbor with fixed ratio. Full matching method was also tried with both logit and probit. To avoid discarding observations and generalize to target population ATE (average treatment effect in the population), we eventually chose optimal full matching method (one-to-many) with logistic regression for propensity score.
Propensity score matching was conducted to balance covariates between two groups, however, at the end for estimating treatment effect, we chose Cox proportional hazards model over other survival analyses so that we again included covariates used in matching to give additional robustness to a small number of imbalances remaining after matching. As a result, we could reduce bias due to residual imbalance, increase the precision of effect estimate and make the result “doubly robust” where we can check if either matching process or outcome model has reduced imbalance correctly.13 We conducted a sensitivity analysis to ensure that the study outcome was robust to hidden bias from unobserved confounders.
All analyses were performed using R v4.2.2 (R-Project, Austria). The MatchIt package Nonparametric Preprocessing for Parametric Causal Inference (Matchlt, United States) was used for propensity score matching.
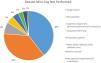
ResultsOf the 1440 patients, 986 (68%) completed the Mini-Cog (cohort A), with 46 (4.7%) positive Mini-Cog (cohort A1) and the remaining 940 (95.3%) having a negative Mini-Cog (cohort A2). The 95% confidence interval for the prevalence of a positive Mini-Cog was 3.4–6.1%. Cohort B includes 454 patients that did not perform the Mini-Cog. Reasons that the Mini-Cog was not completed are shown in Fig. 2. There were 22 patients in cohort B that completed the Mini-Cog but did so as an outpatient prior to admission. Of these 22 patients, 1 (4.5%) had a positive Mini-Cog. Cohort A1 was significantly older, more frequently female, with more chronic kidney disease, heart failure, and chronic obstructive pulmonary disease than the other cohorts. Baseline demographic data for the groups are shown in Table 1.
Clinical characteristics.
| Variables | Cohort A1 (n=46) | Cohort A2 (n=940) | Cohort B (n=454) | P | A1 vs A2P |
|---|---|---|---|---|---|
| Age | 77±12 | 66±13 | 69±14 | <.001 | <.001 |
| Female sex | 27 (59) | 329 (35) | 215 (47) | <.001 | .001 |
| Hypertension | 41 (89) | 746 (79) | 350 (77) | .14 | .13 |
| Hyperlipidemia | 35 (76) | 740 (79) | 319 (70) | .002 | .71 |
| Diabetes | 17 (37) | 304 (32) | 153 (34) | .73 | .52 |
| Coronary artery disease | 29 (63) | 681 (72) | 247 (54) | <.001 | .17 |
| Chronic kidney disease | 30 (65) | 249 (26) | 148 (33) | <.001 | <.001 |
| COPD | 12 (26) | 123 (13) | 89 (20) | <.001 | .02 |
| CHF | 27 (59) | 303 (32) | 128 (28) | <.001 | <.001 |
| CVA | 6 (13) | 66 (7) | 49 (11) | .03 | .13 |
| LVEF | 49±17 | 50±14 | 54±14 | <.001 | .63 |
| Current smoker | 8 (17) | 196 (21) | 76 (17) | .17 | .71 |
| Previous smoker | 21 (46) | 432 (46) | 199 (44) | .75 | 1 |
| Non-smoker | 18 (39) | 312 (33) | 179 (39) | .06 | .42 |
| Minnesota residence | 34 (74) | 661 (70) | 347 (76) | .06 | .74 |
Data are presented as average±standard deviation, or number with percentage shown in parenthesis. A P-value≤.05 was considered statistically significant. CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction.
On univariate analysis cohort A1 had significantly higher rates of all-cause readmission compared with cohorts A2 and B (63% vs 44% and 47%; P=.02), and significantly higher mortality (28% vs 9% vs 15%; P<.001) (Table 2).
Outcome data.
| Variables | Cohort A1 (n=46) | Cohort A2 (n=940) | Cohort B (n=454) | P | A1 vs A2P |
|---|---|---|---|---|---|
| LOS index admission (days) | 6±5 | 5±4 | 5±4 | .25 | .10 |
| Follow-up duration (months) | 11.5±6.4 | 12.4±5.2 | 11.8±5.7 | .10 | .25 |
| Incomplete follow-up | 3 (6.5) | 119 (12.6) | 50 (10.9) | .34 | .35 |
| 30-Day ED visit | 13 (28) | 198 (21) | 81 (18) | .14 | .26 |
| 30-Day cardiac readmission | 9 (20) | 122 (13) | 50 (11) | .20 | .18 |
| 30-Day all-cause readmission | 12 (26) | 156 (17) | 70 (15) | .17 | .10 |
| Cardiac readmission prior to censure | 21 (46) | 309 (33) | 140 (31) | .12 | .07 |
| All-cause readmission prior to censure | 29 (63) | 409 (44) | 212 (47) | .02 | .009 |
| Death | 13 (28) | 80 (9) | 68 (15) | <.001 | <.001 |
| Composite outcome | 34 (74) | 463 (49) | 236 (52) | .004 | .001 |
Data are presented as average±standard deviation, or number with percentage shown in parenthesis. A P-value≤.05 was considered statistically significant. ED, emergency department; LOS, length of stay.
Before matching, there was a large imbalance of covariates between A1 and A2 ranging from 0.1 to 0.87 (absolute value of standardized mean differences) and empirical cumulative density functions max (eCDF max) mostly were ranging from 0.1 to 0.46 (Table 3). Optimal full matching yielded the best balance between two groups, as indicated in Table 4. Sample size remained intact, with the distribution of propensity score shown in Fig. 3. After matching all standardized mean differences and empirical cumulative density functions max (eCDF max) for covariates were all below 0.08 (except one value was 0.11), variance ratios were close to 1. Standardized mean difference plot is indicated in Fig. 4.
Summary of balance for all covariates before matching.
| Covariates | Means treated (positive Mini-Cog) | Means control (negative Mini-Cog) | Standardized mean difference | Variance ratio | eCDF mean | eCDF max |
|---|---|---|---|---|---|---|
| Distance | 0.1067 | 0.0437 | 0.9330 | 1.6208 | 0.3083 | 0.5311 |
| Female sex | 0.5870 | 0.3500 | 0.4812 | 0.2370 | 0.2370 | |
| Male sex | 0.4130 | 0.6500 | −0.4812 | 0.2370 | 0.2370 | |
| Age | 76.6957 | 65.9819 | 0.8754 | 0.8744 | 0.1486 | 0.4615 |
| HTN | 0.8913 | 0.7936 | 0.3138 | 0.0977 | 0.0977 | |
| Hyperlipidemia | 0.7609 | 0.7872 | −0.0618 | 0.0264 | 0.0264 | |
| Diabetes | 0.3696 | 0.3234 | 0.0956 | 0.0462 | 0.0462 | |
| CAD | 0.6304 | 0.7245 | −0.1948 | 0.0940 | 0.0940 | |
| CKD | 0.6522 | 0.2649 | 0.8131 | 0.3873 | 0.3873 | |
| COPD | 0.2609 | 0.1309 | 0.2961 | 0.1300 | 0.1300 | |
| CHF | 0.5870 | 0.3223 | 0.5374 | 0.2646 | 0.2646 | |
| CVA | 0.1304 | 0.0702 | 0.1788 | 0.0602 | 0.0602 | |
| Current smoking | 0.1739 | 0.2085 | −0.0913 | 0.0346 | 0.0346 |
CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; eCDF, empirical cumulative density functions; HTN, hypertension.
Summary of balance for all covariates after optimal full matching.
| Variables | Means treated (positive Mini-Cog) | Means control (negative Mini-Cog) | Standardized mean difference | Variance ratio | eCDF mean | eCDF max | Standard pair difference |
|---|---|---|---|---|---|---|---|
| Distance | 0.1067 | 0.1065 | 0.0041 | 1.0220 | 0.0080 | 0.0652 | 0.0263 |
| Female sex | 0.5870 | 0.5434 | 0.0885 | 0.0436 | 0.0436 | 0.7454 | |
| Male sex | 0.4130 | 0.4566 | −0.0885 | 0.0436 | 0.0436 | 0.7454 | |
| Age | 76.6957 | 76.0574 | 0.0521 | 1.1136 | 0.0321 | 0.1163 | 0.7816 |
| HTN | 0.8913 | 0.8872 | 0.0131 | 0.0041 | 0.0041 | 0.9809 | |
| Hyperlipidemia | 0.7609 | 0.7364 | 0.0573 | 0.0244 | 0.0244 | 1.0176 | |
| Diabetes | 0.3696 | 0.3859 | −0.0338 | 0.0163 | 0.0163 | 0.8089 | |
| CAD | 0.6304 | 0.5953 | 0.0728 | 0.0351 | 0.0351 | 0.8309 | |
| CKD | 0.6522 | 0.6668 | −0.0308 | 0.0147 | 0.0147 | 0.4043 | |
| COPD | 0.2609 | 0.2672 | −0.0144 | 0.0063 | 0.0063 | 0.4167 | |
| CHF | 0.5870 | 0.6036 | −0.0339 | 0.0167 | 0.0167 | 0.6763 | |
| CVA | 0.1304 | 0.1144 | 0.0475 | 0.0160 | 0.0160 | 0.6634 | |
| Current smoking | 0.1739 | 0.2045 | −0.0806 | 0.0306 | 0.0306 | 1.2434 |
CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; eCDF, empirical cumulative density functions; HTN, hypertension.
Survival curves analyzing freedom from the composite outcome of readmission plus death for matched cohorts are shown in Fig. 5. At the 3-month follow-up period in Cox proportional hazards model, cohort A1 had significantly more readmission and death compared to cohort A2 (P=.002). That is, at a given instant in time a patient with positive Mini-Cog is 93.7% times as likely to die or get readmitted to hospital as a patient with negative Mini-Cog adjusting for age, gender and 9 different co-morbidities (95% confidence interval, 1.262–2.974). The result for the entire follow-up period was not significant.
Assessing the Mini-Cog score as a continuous variable revealed progressive increases in death and the composite of death and readmission, along with progressive, significant increases in age, hypertension, chronic kidney disease, diabetes mellitus, and cerebrovascular accident (Tables 5 and 6).
Clinical characteristics.
| Variables | Mini-Cog 5 (n=558) | Mini-Cog 4 (n=251) | Mini-Cog 3 (n=131) | Mini-Cog 2 (n=32) | Mini-Cog 1 (n=11) | Mini-Cog 0 (n=3) | P |
|---|---|---|---|---|---|---|---|
| Age | 64±13 | 67±13 | 72±13 | 75±14 | 78±5 | 84±6 | < .001 |
| Female sex | 191 (34) | 77 (31) | 61 (47) | 19 (59) | 6 (55) | 2 (67) | < .001 |
| Hypertension | 421 (75) | 212 (84) | 113 (86) | 28 (88) | 10 (91) | 3 (100) | .01 |
| Hyperlipidemia | 440 (79) | 202 (80) | 98 (75) | 23 (72) | 9 (82) | 3 (100) | .86 |
| Diabetes | 175 (31) | 78 (31) | 51 (39) | 6 (19) | 9 (82) | 2 (67) | .009 |
| Coronary artery disease | 411 (74) | 182 (73) | 88 (67) | 19 (59) | 9 (82) | 1 (33) | .44 |
| Chronic kidney disease | 114 (20) | 876 (34) | 49 (37) | 20 (63) | 9 (82) | 1 (33) | NaN |
| COPD | 58 (10) | 37 (15) | 28 (21) | 8 (25) | 4 (36) | 0 | .005 |
| CHF | 145 (26) | 98 (39) | 60 (46) | 17 (53) | 9 (82) | 1 (33) | < .001 |
| CVA | 29 (5) | 22 (9) | 15 (11) | 3 (9) | 1 (9) | 2 (67) | .01 |
| LVEF | 51±14 | 48±15 | 50±15 | 48±17 | 51±15 | 60 | .09 |
| Current smoker | 105 (19) | 68 (27) | 23 (18) | 6 (19) | 2 (18) | 0 | .17 |
| Previous smoker | 263 (47) | 103 (41) | 66 (50) | 15 (47) | 5 (45) | 1 (33) | .55 |
| Non-smoker | 190 (34) | 80 (32) | 42 (32) | 11 (34) | 4 (36) | 2 (67) | .98 |
| Minnesota residence | 385 (69) | 182 (73) | 94 (72) | 25 (78) | 6 (55) | 3 (100) | .78 |
Data are presented as average±standard deviation, or number with percentage shown in parenthesis. A P-value≤.05 was considered statistically significant. COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; NaN, not a number.
Outcome data.
| Variables | Mini-Cog 5 (n=558) | Mini-Cog 4 (n=251) | Mini-Cog 3 (n=131) | Mini-Cog 2 (n=32) | Mini-Cog 1 (n=11) | Mini-Cog 0 (n=3) | P |
|---|---|---|---|---|---|---|---|
| LOS index admission (days) | 5±4 | 5±4 | 7±7 | 7±6 | 5±3 | 5±2 | < .001 |
| Follow-up duration (months) | 12.5±5.1 | 12.2±5.5 | 12.4±5.3 | 11.7±6.4 | 11.2±7.4 | 10±1.6 | .81 |
| 30-Day ED visit | 99 (18) | 57 (23) | 31 (24) | 8 (25) | 4 (36) | 1 (33) | .45 |
| 30-Day cardiac readmission | 72 (13) | 33 (13) | 17 (13) | 6 (19) | 3 (27) | 0 | .93 |
| 30-Day all-cause readmission | 88 (16) | 42 (17) | 26 (20) | 8 (25) | 4 (36) | 0 | .58 |
| Cardiac readmission prior to censure | 178 (32) | 85 (34) | 46 (35) | 14 (44) | 6 (55) | 1 (33) | .62 |
| All-cause readmission prior to censure | 231 (41) | 110 (44) | 68 (52) | 19 (59) | 7 (64) | 3 (100) | .08 |
| Death | 31 (6) | 29 (12) | 20 (15) | 8 (25) | 4 (36) | 1 (33) | < .001 |
| Composite outcome | 255 (46) | 131 (52) | 77 (59) | 22 (69) | 9 (82) | 3 (100) | .005 |
Data are presented as average±standard deviation, or number with percentage shown in parenthesis. A P-value≤.05 was considered statistically significant. ED, emergency department; LOS, length of stay.
Sub-group analysis found 9.5% of those ≥70 years of age had cognitive impairment. Patients≥70 years of age, with a positive Mini-Cog had higher rates of all-cause readmission and death compared to patients≥70 years of age with a negative Mini-Cog, but significant differences between the cohorts remained. Of those ≥80 years of age, 12.3% had cognitive impairment. The baseline characteristics were very similar between those with and without a positive Mini-Cog and ≥80 years of age, with no difference in readmission or death. Of the 590 patients<70 years of age, 8 (1.3%) had cognitive impairment.
Within cohort B were 35 (8%) patients that either had a history of dementia/cognitive impairment or had a Montreal Cognitive Assessment (MoCA) administered by an occupational therapist that identified cognitive impairment during their index admission. During the follow-up period 12 (34%) of these patients died and 21 (60%) met the combined end-point of readmission+death.
Finally, we conducted sensitivity analysis for optimal full matching. We used Huber–Maritz M-statistics with Γ=1 and P=.0069, which means the outcome and study conclusion was robust to hidden bias from an unobserved confounder.
DiscussionIn our phase I cardiac rehabilitation cohort, poor performance on the Mini-Cog identified an elderly group with significantly increased rates of readmission and death. This poorly performing Mini-Cog group was also found to be an older patient population with multiple co-morbidities.
A positive Mini-Cog was found to be associated with increased absolute levels of death, cardiac readmission and all-cause readmission, with the composite end-point of all-cause readmission+death reaching statistical significance (P=.05). Cox proportional hazards model with survival curves found cohort A1 to have significantly increased readmission+death at 3 months compared to cohort A2.
These results are consistent with previous publications that have found that cognitive impairment is associated with increased readmission rates and poorer survival in heart failure patients.8–12 A 2015 study that looked specifically at heart failure patients with a positive Mini-Cog found significantly increased rates of readmission and death.9 Interestingly, if the patients were discharged to a long-term care facility then their risk of readmission and death decreased.8 Another study found cognitive impairment to be an independent predictor of mortality in veterans with heart failure.14
The prevalence of 4.6% that we observed in our cohort is a relatively novel finding, as we are unaware of any other publication that evaluated the rate of cognitive impairment in a large, consecutive cardiopulmonary rehabilitation cohort. A 2017 publication from Germany found 36.7% of younger cardiac rehabilitation patients (average age 54.5 years), with a diagnosis of coronary artery disease, had a MoCA score below 26, which was used as the threshold for cognitive impairment.15 This rate of cognitive impairment is significantly higher than what we observed in our cohort, despite their cohort being significantly younger than ours. A potential explanation for the stark difference in the rate of cognitive impairment between our data and this publication is their use of MoCA to assess for cognitive impairment and our use of the Mini-Cog. The MoCA has been shown to have much lower specificity than the Mini-Mental State Examination (MMSE) for detecting cognitive impairment post-stroke.16 Interestingly, the MMSE was used as the gold-standard to establish the efficacy of the Mini-Cog for diagnosing cognitive impairment.5 It is also worth noting that 5% of the German cohort had more severe cognitive impairment, which could potentially be a group more similar to our cohort A1.15
The prevalence of cognitive impairment increased with age in our cohort, with 9.5% of those 70 or older and 12.3% of those 80 and older having a positive Mini-Cog test. This is consistent with previous publications, including a large retrospective series that found 11.6% of patients≥75 years of age to have undiagnosed cognitive impairment.2–5,8–11 Cognitive impairment was uncommon in patients<70 years of age in our cohort. With consideration of this finding, we have decided to no longer administer the Mini-Cog to phase I cardiopulmonary rehabilitation patients younger than 65 years of age.
The simple incorporation of the Mini-Cog into phase I cardiac rehabilitation provides prognostic information for patients that have a positive assessment. Identifying individuals with cognitive impairment provides an opportunity to intervene, which may slow the progression of their cognitive impairment and improve outcomes. The targets of interventions include reducing alcohol consumption, smoking cessation, treating vascular risk factors, cognitive activities (games, music, reading, etc.), cardiovascular exercise, strength training and even adopting a Mediterranean diet.17,18 Poor cardiorespiratory fitness is also associated with cognitive dysfunction, with cardiac rehabilitation being associated with improvements in cognition.19–21 Thus, referral to phase II cardiac rehabilitation will allow them to derive benefit that may improve their cognitive function, in addition to providing the typical benefits of cardiac rehabilitation – improved exercise capacity, improved quality of life, improved cardiovascular risk factor profile, and improved ability to perform activities of daily living.19–26 Even if these patients are unable to attend all prescribed sessions of cardiac rehab publications have identified the incremental benefit of individual sessions on readmission rates.27,28 Future retrospective studies could validate our findings in a larger database, while future prospective, randomized studies could evaluate interventions to reduce mortality and morbidity in patients identified as having cognitive impairment on the Mini-Cog assessment.
LimitationsThe cohort with a positive Mini-Cog (cohort A1) is much smaller than the other cohorts, which weakens the strength of our comparisons. While the Mini-Cog test was administered prospectively the data was collected retrospectively. This creates the possibility of selection bias. There were 172 (12%) patients that had incomplete follow-up data. While Care Everywhere records were used to collect information about readmissions occurring outside the HealthPartners system, incomplete readmission data from outside hospitals likely results in an underestimation of the actual readmission rate. We believe that death records for Minnesota residents is accurate, but mortality for Wisconsin residents, as well as the small percentage of patients from outside Minnesota or Wisconsin (2%), is likely an underestimation. We do not have specific information on each patient's Mini-Cog assessment, only the composite score.
ConclusionsThe Mini-Cog assessment can be incorporated into an inpatient cardiac rehabilitation program and will identify cognitive impairment in approximately 10% of those ≥70 years of age. Poor performance on the Mini-Cog identified an older group with multiple co-morbidities that had significantly increased rates of readmission and death at 3-months follow-up.
- -
The Mini-Cog test is a quick and effective tool for identifying cognitive dysfunction.
- -
Cognitive impairment is common in patients with heart failure.
- -
A positive Mini-Cog was associated with increased rates of readmission and death in a heart failure cohort.
- -
This is the first publication to incorporate the mini-cog mental examination into a phase I cardiac rehabilitation program.
- -
We found 5% of our study population to have a positive mini-cog, consistent with cognitive impairment, including 10% of patients 70 years of age or older.
- -
The group with a positive Mini-Cog had significantly increased readmission or death at 3 months.
The authors received no financial support for their research, authorship, and/or publication of this article.
Authors’ contributionC. House collected data, analyzed data, and drafted the manuscript; H. Dang performed the statistical analysis; K. Moriarty critically revised the manuscript; W. Nelson conceptualized the study and critically revised the manuscript. All authors revised and accepted the manuscript.
Conflicts of interestThe authors declare that there is no conflict of interest.