Iron deficiency (ID) is common in heart failure (HF) patients and is linked to exercise impairment, worse quality of life, and HF hospitalisation. Clinical practice guidelines recommend checking and correcting ID with ferric carboxymaltose (FCM). However, there is a lack of evidence in patients with left ventricular ejection fraction (LVEF) >40%.
MethodsWe included all HF outpatients treated with FCM after ID diagnosis (ferritin <100ng/mL or ferritin 100–299ng/mL and transferrin saturation <20%). We analysed clinical and analytical parameters before FCM administration and at 3 months according to LVEF: preserved (>50%), mildly reduced (41–49%), and reduced (<40%).
ResultsWe included 235 patients (51.5% female) aged 73.5±10.7 years. Ninety-six patients have reduced LVEF (40.8%), 41 mildly reduced (17.4%), and 98 preserved (41.7%). Patients with preserved LVEF have more anaemia (42.6% vs 26.8% vs 52.6%; P=.02). Less than 50% of patients received the correct dose of FCM, especially patients with preserved LVEF (P=.004). One patient (0.4%) presented a local exanthema with no other adverse effects. At 3 months, all analytical parameters significantly improved, except haemoglobin (12.9 vs 13.0mg/dL; P=.95) and natriuretic peptides (3261 vs 3471pg/mL; P=.56) in mildly reduced LVEF patients. The functional class did not improve in preserved LVEF patients, but it did in the rest.
ConclusionsFCM is safe and effective in correcting ID in HF patients regardless of LVEF. Natriuretic peptides are reduced in all patients except those with mildly reduced LVEF. Functional class improvement is less likely in patients with preserved LVEF.
El déficit de hierro (DH) es frecuente en pacientes con insuficiencia cardiaca y se relaciona con una reducción en la capacidad del ejercicio, peor calidad de vida y hospitalizaciones por dicha insuficiencia. Las guías de práctica clínica recomiendan detectar y corregir el DH con carboximaltosa férrica (CMF). Sin embargo, existe poca evidencia en pacientes con fracción de eyección del ventrículo izquierdo (FEVI) >40%.
MétodosSe incluyó a todos los pacientes ambulatorios con insuficiencia cardiaca tratados con CMF tras el diagnóstico de DH (ferritina <100ng/ml o ferritina 100-299ng/ml y saturación de transferrina <20%). Se analizaron los parámetros clínicos y analíticos antes de la administración de CMF y a los 3 meses según la FEVI: conservada (>50%), levemente reducida (41-49%) y reducida (<40%).
ResultadosFueron incluidos 235 pacientes (51,5% mujeres) con una edad de 73,5±10,7 años; 96 pacientes tenían FEVI reducida (40,8%), 41 ligeramente reducida (17,4%) y 98 conservada (41,7%). Los pacientes con FEVI conservada tenían más anemia (42,6 frente a 26,8 frente a 52,6%; p=0,02). Menos del 50% de los pacientes recibieron la dosis correcta de CMF, especialmente los pacientes con FEVI conservada (p=0,004). Un paciente (0,4%) presentó un exantema local, sin otros efectos adversos. A los 3 meses, todos los parámetros analíticos mejoraron significativamente, excepto la hemoglobina (12,9 frente a 13,0mg/dl; p=0,95) y los péptidos natriuréticos (3.261 frente a 3.471pg/ml; p=0,56) en los pacientes con FEVI levemente reducida. La clase funcional no mejoró en los pacientes con FEVI conservada, pero sí en el resto.
ConclusionesEl tratamiento con CMF es seguro y eficaz para corregir el DH en pacientes con insuficiencia cardiaca con independencia de la FEVI. Los péptidos natriuréticos se reducen en todos los pacientes excepto en aquellos con FEVI levemente reducida. La mejoría de la clase funcional es menos probable en los pacientes con FEVI conservada.
Iron deficiency (ID) affects 55–80% of heart failure (HF) patients regardless of left ventricular ejection fraction (LVEF). ID has been linked to reduced exercise capacity, HF hospitalisation, and worse prognosis, independently of anaemia or other comorbidities.1–3
Clinical practice guidelines recommended periodically screening ID in HF patients and correcting with intravenous ferric carboxymaltose (FCM).4,5 Based on FAIR-HF6 and CONFIRM-HF7 trials, FCM is indicated in HF patients with LVEF <45% to improve HF symptoms, exercise capacity, and quality of life.6,7 Recently, the AFFIRM-HF8 trial extended the benefit to patients recently hospitalised for HF and LVEF <50% to reduce the risk of HF hospitalization.7 Thus, the evidence in HF with reduced LVEF (HfrEF) is strong, but there is a lack of evidence in HF with mildly reduced LVEF (HfmrEF) and preserved LVEF (HfpEF), considering that the characteristics and outcomes of HF patients are different according to LVEF.9,10
Recent studies showed that ID is linked to a higher risk for worsening HF and functional deterioration in all-range LVEF, and its correction could improve exercise capacity and functional outcomes.1,11,12 This study aims to evaluate the effectiveness and safety of ID correction with FCM at 3-month follow-up in a cohort of HF outpatients across the whole spectrum of LVEF.
MethodsWe performed a retrospective, unicentric, and observational registry, including all consecutive HF outpatients who received FCM to correct ID between 2015 and 2017 in the HF unit, according to current recommendations.4,13 We included all-range LVEF patients because ID was identified as a relevant parameter in all HF patients,1,14 waiting for the results of the Effect of IV Iron in Patients With Heart Failure With Preserved Ejection Fraction trial (FAIR-HfpEF; NCT03074591). The study complied with the Declaration of Helsinki and was approved by the Hospital Ethics Committee of Hospital Universitario Clínico San Carlos. Due to the retrospective character analysis, obtaining informed consent was unnecessary. Data were recorded in an electronic data database with anonymised patient data. Only the principal investigators can consult the total data of the included patients.
All patients have a chronic history of clinical HF, and the classification of HF was made according to clinical guidelines.5 All the analysis was made according to LVEF: HfrEF (<40%), HfmrEF (41–49%), and HfpEF (≥50%). In case of HfpEF, due to the heterogenity of this population, all of them have HF symptoms, a LVEF ≥50% and raised natriuretic peptides.
Symptomatic patients received FCM when ID was detected, defined as ferritin <100ng/mL (absolute deficiency) or ferritin 100–299ng/mL with transferrin saturation <20% (functional deficiency). Anaemia was defined as haemoglobin (Hb) <12g/dL (female) and <13g/dL (male).4,5 According to the studies with FCM, patients with Hb >15mg/dL were excluded, with no other exclusion criteria. FCM was administered in saline 0.9% (50–150mL) for 15min (maximum 1000mg of FCM a week) in the daily hospital with nurse supervision and 30min of monitoring post-infusion. The dose of FCM was calculated based on weight and Hb: (a) Hb 10–14g/dL and weight <70kg, 1000mg; (b) Hb <10g/dL and weight <70kg, 1500mg; (c) Hb <14g/dL and weight ≥70kg, 2000mg; (d) Hb ≥14g/dL, 500mg
We analysed different variables before FCM infusion: (a) medical history, vital signs, New York Heart Association (NYHA) functional class, HF aetiology, and LVEF (Simpson method by echocardiogram); (b) blood test: hematic and ferric parameters, N-terminal pro-B-type natriuretic peptide (NT-proBNP), renal function, and electrolytes; (c) aspects related to FCM: dose and adverse effects; (d) in the patients that voluntary wanted to answer, the health-related quality of life (HRQoL) with the EQ-5D-3L test15 and the Spanish version of Minnesota Living with Heart Failure Questionnaire (MLHFQ).16,17 We repeated clinical, analytical, and HRQoL tests at 3 months, according to clinical practice guidelines.4,18 At 3 months, all patients were answered about their subjective perception of clinical improvement after FCM administration.
Quantitative variables are expressed as mean (standard deviation) or median [interquartile range] in nonparametric data, and qualitative variables as numbers and percentages. The Student's t-test, or the sum of Wilcoxon ranges in nonparametric data, was used to compare continuous quantitative variables. Categorical variables were compared with the chi-square test and Fisher's exact test. A significance level of 0.05 (bilateral) was established for all statistical tests. The statistical analysis was performed with SPSS 21.0 (IBM, United States).
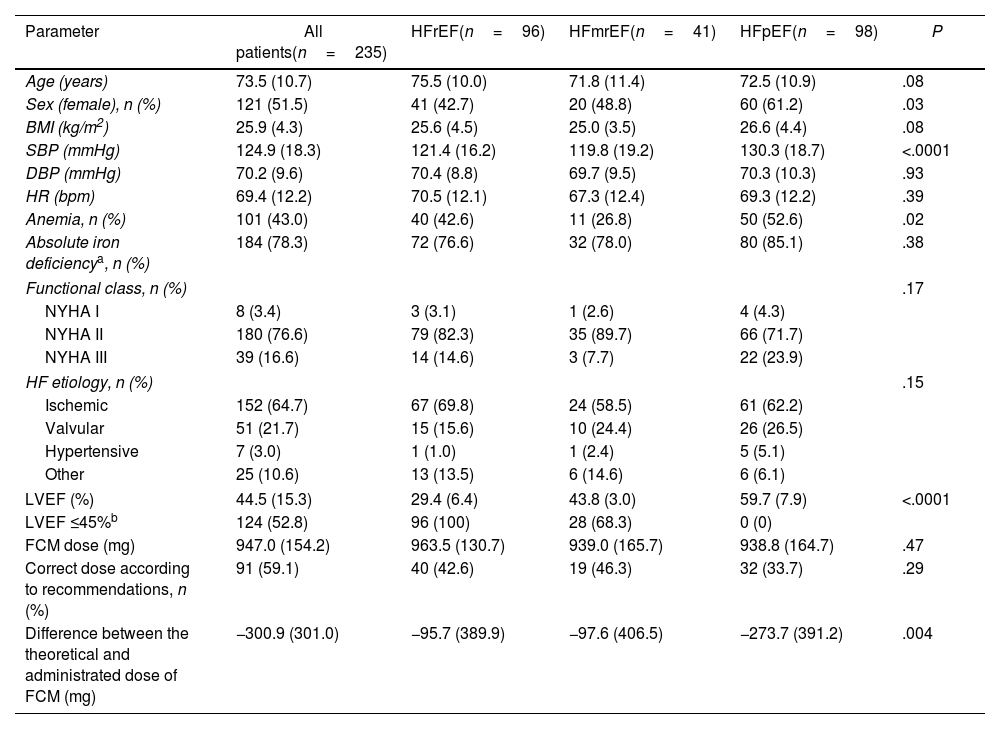
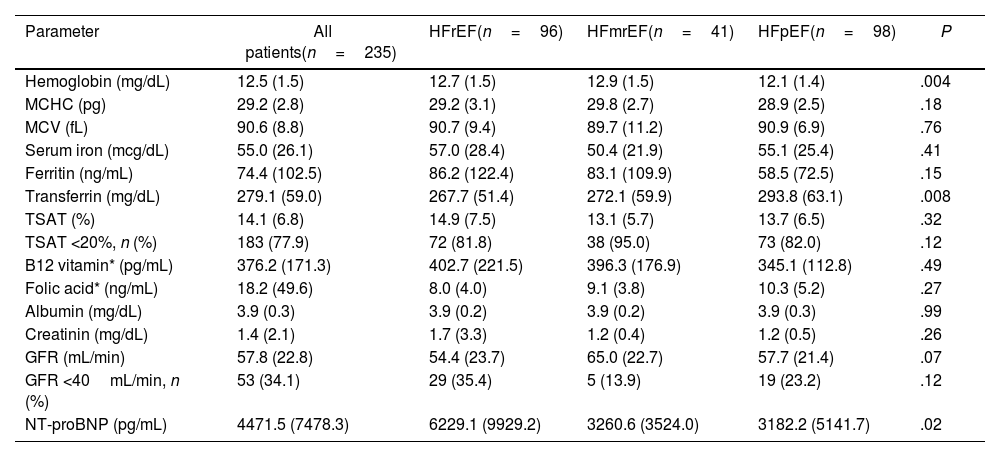
ResultsWe included 235 patients (51.5% female) with a mean age of 73.5 years (SD, 10.7), 50.8% older than 75 years. There were 96 patients with HFrEF (40.8%), 41 with HFmrEF (17.4%), and 98 with HFpEF (41.7%). There were more females in HFmrEF and HFpEF groups, and patients with HFpEF had more anaemia and higher blood pressure (Table 1). There were no differences between groups in HF aetiology, NYHA, or type of ID (absolute or functional). Also, there were no differences in ferric analytical parameters except in transferrin, which was higher in HFpEF. Patients with HFrEF also have significantly higher basal NTproBNP levels (P=.02), being quite similar in HFmrEF and HFpEF groups (Table 2).
Baseline characteristics of HF patients with iron deficiency according to LVEF.
| Parameter | All patients(n=235) | HFrEF(n=96) | HFmrEF(n=41) | HFpEF(n=98) | P |
|---|---|---|---|---|---|
| Age (years) | 73.5 (10.7) | 75.5 (10.0) | 71.8 (11.4) | 72.5 (10.9) | .08 |
| Sex (female), n (%) | 121 (51.5) | 41 (42.7) | 20 (48.8) | 60 (61.2) | .03 |
| BMI (kg/m2) | 25.9 (4.3) | 25.6 (4.5) | 25.0 (3.5) | 26.6 (4.4) | .08 |
| SBP (mmHg) | 124.9 (18.3) | 121.4 (16.2) | 119.8 (19.2) | 130.3 (18.7) | <.0001 |
| DBP (mmHg) | 70.2 (9.6) | 70.4 (8.8) | 69.7 (9.5) | 70.3 (10.3) | .93 |
| HR (bpm) | 69.4 (12.2) | 70.5 (12.1) | 67.3 (12.4) | 69.3 (12.2) | .39 |
| Anemia, n (%) | 101 (43.0) | 40 (42.6) | 11 (26.8) | 50 (52.6) | .02 |
| Absolute iron deficiencya, n (%) | 184 (78.3) | 72 (76.6) | 32 (78.0) | 80 (85.1) | .38 |
| Functional class, n (%) | .17 | ||||
| NYHA I | 8 (3.4) | 3 (3.1) | 1 (2.6) | 4 (4.3) | |
| NYHA II | 180 (76.6) | 79 (82.3) | 35 (89.7) | 66 (71.7) | |
| NYHA III | 39 (16.6) | 14 (14.6) | 3 (7.7) | 22 (23.9) | |
| HF etiology, n (%) | .15 | ||||
| Ischemic | 152 (64.7) | 67 (69.8) | 24 (58.5) | 61 (62.2) | |
| Valvular | 51 (21.7) | 15 (15.6) | 10 (24.4) | 26 (26.5) | |
| Hypertensive | 7 (3.0) | 1 (1.0) | 1 (2.4) | 5 (5.1) | |
| Other | 25 (10.6) | 13 (13.5) | 6 (14.6) | 6 (6.1) | |
| LVEF (%) | 44.5 (15.3) | 29.4 (6.4) | 43.8 (3.0) | 59.7 (7.9) | <.0001 |
| LVEF ≤45%b | 124 (52.8) | 96 (100) | 28 (68.3) | 0 (0) | |
| FCM dose (mg) | 947.0 (154.2) | 963.5 (130.7) | 939.0 (165.7) | 938.8 (164.7) | .47 |
| Correct dose according to recommendations, n (%) | 91 (59.1) | 40 (42.6) | 19 (46.3) | 32 (33.7) | .29 |
| Difference between the theoretical and administrated dose of FCM (mg) | −300.9 (301.0) | −95.7 (389.9) | −97.6 (406.5) | −273.7 (391.2) | .004 |
BMI: body mass index; BPM: beats per minute; DBP: diastolic blood pressure; FCM: ferric carboxymaltose; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; HR: heart rate; ID: iron deficiency; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SBP: systolic blood pressure.
Analytical parameters of HF patients with iron deficiency according to LVEF.
| Parameter | All patients(n=235) | HFrEF(n=96) | HFmrEF(n=41) | HFpEF(n=98) | P |
|---|---|---|---|---|---|
| Hemoglobin (mg/dL) | 12.5 (1.5) | 12.7 (1.5) | 12.9 (1.5) | 12.1 (1.4) | .004 |
| MCHC (pg) | 29.2 (2.8) | 29.2 (3.1) | 29.8 (2.7) | 28.9 (2.5) | .18 |
| MCV (fL) | 90.6 (8.8) | 90.7 (9.4) | 89.7 (11.2) | 90.9 (6.9) | .76 |
| Serum iron (mcg/dL) | 55.0 (26.1) | 57.0 (28.4) | 50.4 (21.9) | 55.1 (25.4) | .41 |
| Ferritin (ng/mL) | 74.4 (102.5) | 86.2 (122.4) | 83.1 (109.9) | 58.5 (72.5) | .15 |
| Transferrin (mg/dL) | 279.1 (59.0) | 267.7 (51.4) | 272.1 (59.9) | 293.8 (63.1) | .008 |
| TSAT (%) | 14.1 (6.8) | 14.9 (7.5) | 13.1 (5.7) | 13.7 (6.5) | .32 |
| TSAT <20%, n (%) | 183 (77.9) | 72 (81.8) | 38 (95.0) | 73 (82.0) | .12 |
| B12 vitamin* (pg/mL) | 376.2 (171.3) | 402.7 (221.5) | 396.3 (176.9) | 345.1 (112.8) | .49 |
| Folic acid* (ng/mL) | 18.2 (49.6) | 8.0 (4.0) | 9.1 (3.8) | 10.3 (5.2) | .27 |
| Albumin (mg/dL) | 3.9 (0.3) | 3.9 (0.2) | 3.9 (0.2) | 3.9 (0.3) | .99 |
| Creatinin (mg/dL) | 1.4 (2.1) | 1.7 (3.3) | 1.2 (0.4) | 1.2 (0.5) | .26 |
| GFR (mL/min) | 57.8 (22.8) | 54.4 (23.7) | 65.0 (22.7) | 57.7 (21.4) | .07 |
| GFR <40mL/min, n (%) | 53 (34.1) | 29 (35.4) | 5 (13.9) | 19 (23.2) | .12 |
| NT-proBNP (pg/mL) | 4471.5 (7478.3) | 6229.1 (9929.2) | 3260.6 (3524.0) | 3182.2 (5141.7) | .02 |
GFR: glomerular filtrated rate; Hb: haemoglobin; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; LVEF: left ventricular ejection fraction; MCHC: mean corpuscular haemoglobin concentration; MCV: mean corpuscular volume; TSAT: transferrin saturation.
Two hundred and six patients (87.7%) answered the tests before FCM administration. There were differences in basal EQ-5D index between groups (Table 1 of the supplementary data), with worse punctuations in the HFpEF group (0.7±0.2 in HFrEF, 0.7±0.3 in HFmrEF, 0.6±0.2 in HFpEF; P=.01), especially in the question of activities, with fewer patients without any problem to do activities in HFpEF (56.6% in HFrEF, 58.3% HFmrEF and 35.6% in HFpEF; P=.049).
The mean dose of FCM was 947.0mg (SD, 154.2), with <50% of patients receiving the correct dose according to recommendations, especially in HFpEF patients (P=.004). One patient (0.4%) with HFrEF presented a local exanthema resolved with oral antihistaminics and corticosteroids. There were no other adverse effects.
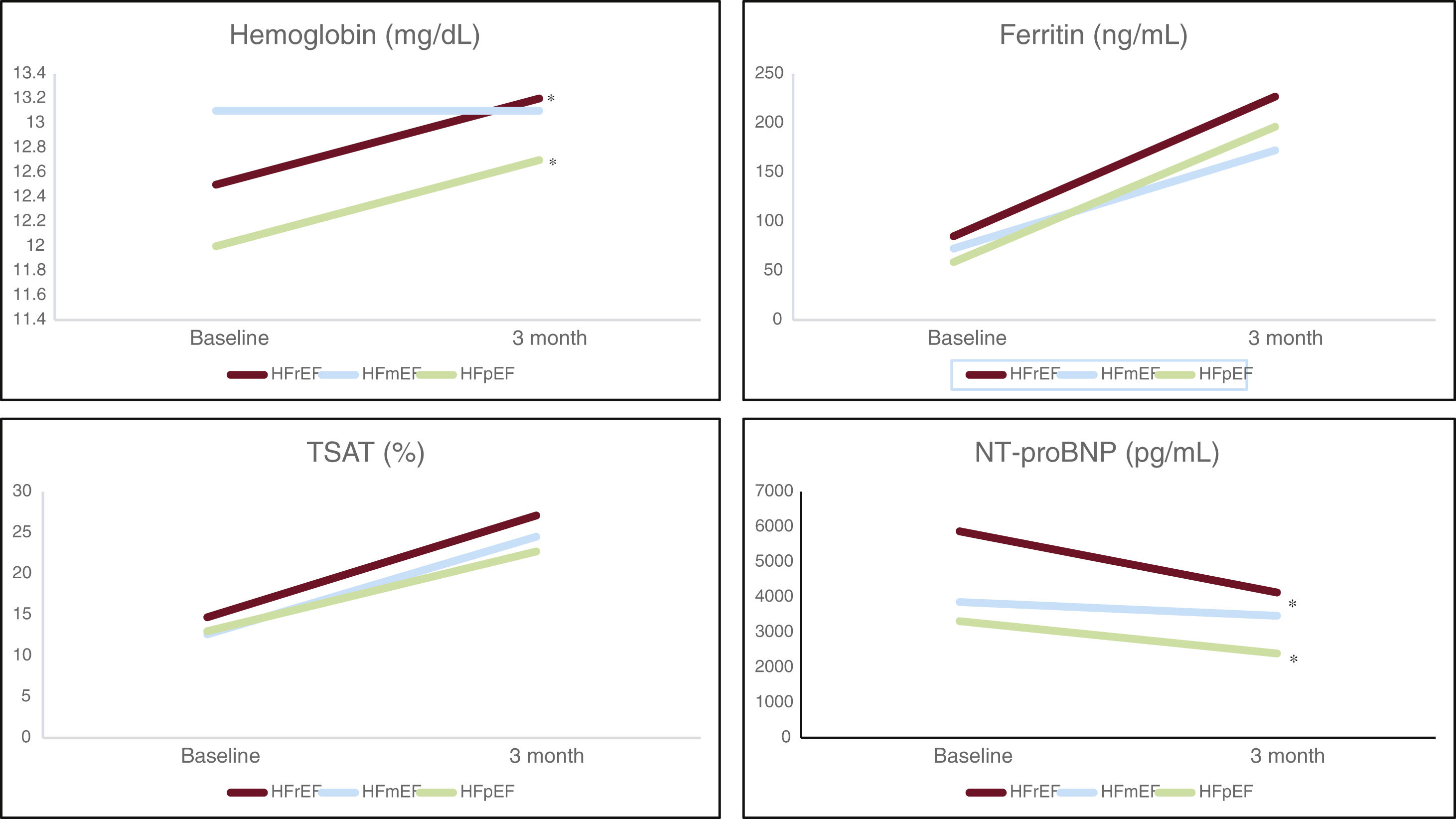
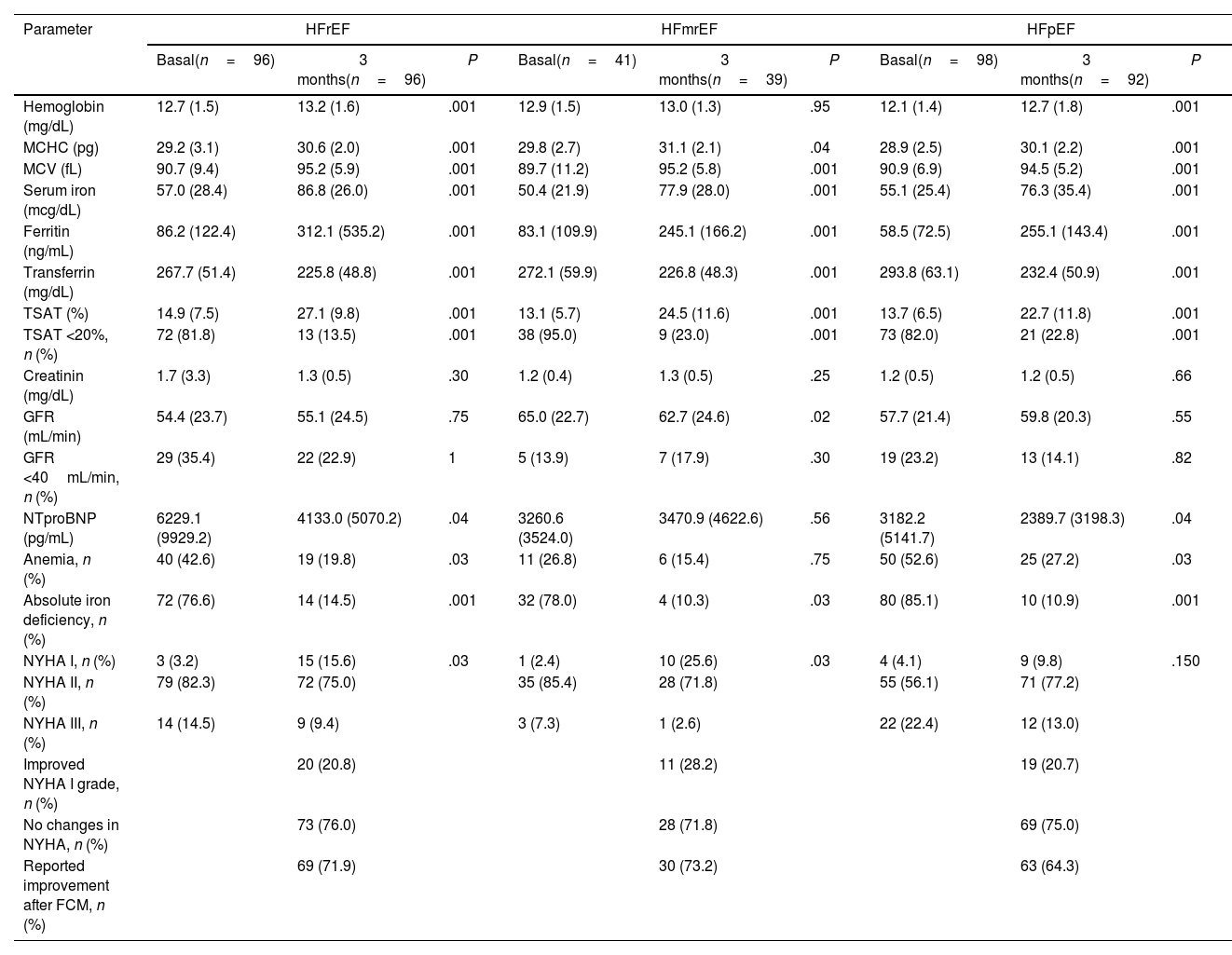
The mean follow-up time after FCM infusion was 89.8 days (SD, 2.9), with HFrEF and HFpEF patients improving ferric parameters and haemoglobin (Table 3). In HFmrEF, the percentage of patients with anaemia was similar to before FCM administration, and haemoglobin levels did not significantly change (12.9 vs 13.0; P=.95). NTproBNP levels decreased in HFrEF and HFpEF patients, and the NYHA functional class improved in HFrEF and HFmrEF ones (Fig. 1).
Clinical and analytical parameters at 3-month follow-up according to LVEF.
| Parameter | HFrEF | HFmrEF | HFpEF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal(n=96) | 3 months(n=96) | P | Basal(n=41) | 3 months(n=39) | P | Basal(n=98) | 3 months(n=92) | P | |
| Hemoglobin (mg/dL) | 12.7 (1.5) | 13.2 (1.6) | .001 | 12.9 (1.5) | 13.0 (1.3) | .95 | 12.1 (1.4) | 12.7 (1.8) | .001 |
| MCHC (pg) | 29.2 (3.1) | 30.6 (2.0) | .001 | 29.8 (2.7) | 31.1 (2.1) | .04 | 28.9 (2.5) | 30.1 (2.2) | .001 |
| MCV (fL) | 90.7 (9.4) | 95.2 (5.9) | .001 | 89.7 (11.2) | 95.2 (5.8) | .001 | 90.9 (6.9) | 94.5 (5.2) | .001 |
| Serum iron (mcg/dL) | 57.0 (28.4) | 86.8 (26.0) | .001 | 50.4 (21.9) | 77.9 (28.0) | .001 | 55.1 (25.4) | 76.3 (35.4) | .001 |
| Ferritin (ng/mL) | 86.2 (122.4) | 312.1 (535.2) | .001 | 83.1 (109.9) | 245.1 (166.2) | .001 | 58.5 (72.5) | 255.1 (143.4) | .001 |
| Transferrin (mg/dL) | 267.7 (51.4) | 225.8 (48.8) | .001 | 272.1 (59.9) | 226.8 (48.3) | .001 | 293.8 (63.1) | 232.4 (50.9) | .001 |
| TSAT (%) | 14.9 (7.5) | 27.1 (9.8) | .001 | 13.1 (5.7) | 24.5 (11.6) | .001 | 13.7 (6.5) | 22.7 (11.8) | .001 |
| TSAT <20%, n (%) | 72 (81.8) | 13 (13.5) | .001 | 38 (95.0) | 9 (23.0) | .001 | 73 (82.0) | 21 (22.8) | .001 |
| Creatinin (mg/dL) | 1.7 (3.3) | 1.3 (0.5) | .30 | 1.2 (0.4) | 1.3 (0.5) | .25 | 1.2 (0.5) | 1.2 (0.5) | .66 |
| GFR (mL/min) | 54.4 (23.7) | 55.1 (24.5) | .75 | 65.0 (22.7) | 62.7 (24.6) | .02 | 57.7 (21.4) | 59.8 (20.3) | .55 |
| GFR <40mL/min, n (%) | 29 (35.4) | 22 (22.9) | 1 | 5 (13.9) | 7 (17.9) | .30 | 19 (23.2) | 13 (14.1) | .82 |
| NTproBNP (pg/mL) | 6229.1 (9929.2) | 4133.0 (5070.2) | .04 | 3260.6 (3524.0) | 3470.9 (4622.6) | .56 | 3182.2 (5141.7) | 2389.7 (3198.3) | .04 |
| Anemia, n (%) | 40 (42.6) | 19 (19.8) | .03 | 11 (26.8) | 6 (15.4) | .75 | 50 (52.6) | 25 (27.2) | .03 |
| Absolute iron deficiency, n (%) | 72 (76.6) | 14 (14.5) | .001 | 32 (78.0) | 4 (10.3) | .03 | 80 (85.1) | 10 (10.9) | .001 |
| NYHA I, n (%) | 3 (3.2) | 15 (15.6) | .03 | 1 (2.4) | 10 (25.6) | .03 | 4 (4.1) | 9 (9.8) | .150 |
| NYHA II, n (%) | 79 (82.3) | 72 (75.0) | 35 (85.4) | 28 (71.8) | 55 (56.1) | 71 (77.2) | |||
| NYHA III, n (%) | 14 (14.5) | 9 (9.4) | 3 (7.3) | 1 (2.6) | 22 (22.4) | 12 (13.0) | |||
| Improved NYHA I grade, n (%) | 20 (20.8) | 11 (28.2) | 19 (20.7) | ||||||
| No changes in NYHA, n (%) | 73 (76.0) | 28 (71.8) | 69 (75.0) | ||||||
| Reported improvement after FCM, n (%) | 69 (71.9) | 30 (73.2) | 63 (64.3) | ||||||
GFR: glomerular filtrated rate; FCM: ferric carboxymaltose; Hb: haemoglobin; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; LVEF: left ventricular ejection fraction; MCHC: mean corpuscular haemoglobin concentration; MCV: mean corpuscular volume; NYHA: New York Heart Association; TSAT: transferrin saturation.
Changes in analytical parameters after FCM administration according to LVEF. FCM: ferric carboxymaltose; HFmrEF: heart failure with a mildly reduced left ventricular ejection fraction; HfpEF: heart failure with a preserved left ventricular ejection fraction; HfrEF: heart failure with a reduced left ventricular ejection fraction; LVEF, left ventricular ejection fraction; TSAT, transferrin saturation.
Only 52 patients (22.1%) completed the HRQoL tests at 3 months (23 in HFrEF, 11 in HFmrEF, and 18 in HFpEF). There was no improvement in any test in HFmrEF patients. In HFrEF, there was only an improvement in question 6 in the Minnesota test (P=.009). HFpEF patients only improve in question 2 (P=.002) and 5 (P=.005) of the Minnesota test and the physical dimension in MLHFQ (P=.031).
DiscussionThe main findings of our study are: (a) FCM is effective in correcting ID regardless of LVEF; (b) HFpEF patients were less likely to improve functional class and report clinical improvement; (c) NT-proBNP is reduced at 3 months of FCM administration, even in the HFpEF group; (d) administration of FCM is safe in all patients with HF.
ID affects more than half of the patients with HF, regardless of LVEF.3,19 In the CARENFER study, the prevalence was a little higher in HFpEF patients than in HFmrEF (57.5% vs 47.4%) or HFrEF ones (44.3%),20 and also Martens et al. reported a higher prevalence in HFpEF than in HFmrEF (64% vs 61%) and HFrEF (50%).11 ID has been linked to impaired functional capacity and worse exercise capacity in all HF patients, and probably also in clinical outcomes, especially HF hospitalization.11 The myocardium of HF patients with ID has a lower concentration of iron than no HF patients,21 affecting cardiomyocyte contraction and relaxation,22 explaining the symptoms of HF patients with ID. Different studies observed mitochondrial dysfunction in ID patients, with lower mRNA levels of mitochondrial ferritin and other proteins linked to iron regulation and endothelial function, impacting prognosis, especially in HFrEF patients.1,3,23,24 In our study, less than 5% of the patients were asymptomatic, and the most symptomatic ones were HFpEF, which probably have more diastolic dysfunction and comorbidities (i.e. anaemia), affecting the functional class. Ferric parameters were quite similar between groups in our study, except for anaemia, which is more frequent in HFpEF patients.
FCM is indicated to correct ID in chronic symptomatic outpatients with LVEF <45% to improve HF symptoms, exercise capacity, and quality of life,5 with constated evidence of myocardial iron repletion after FCM administration.25 The evidence with FCM is limited in chronic patients with HFmrEF and HFpEF. López-Vilella et al., in a study with 565 outpatients with HFpEF, showed that FCM administration improves all analytical parameters,26 similar to ours. However, the benefit of FCM in functional class improvement is controversial in patients with LVEF >40%.1,27,14 A study showed a slight improvement in NYHA class in HFpEF after FCM administration, but it did not seem clinically relevant (from a median of 2.4 to 1.9).26 Mollace et al. also showed an improvement in exercise capacity after FCM, and it was more likely in HFpEF patients with more affected diastolic function due to enhancing endothelial function and reducing the production of oxygen radical species.28 In our study, HFpEF patients, who have worse basal NYHA and HRQoL test punctuations, did not significantly improve NYHA class or refer clinical improvement after FCM infusion compared to the other groups. The different phenotypes of HFpEF patients, with a high prevalence of other comorbidities, such as anaemia of pulmonary hypertension, can interfere with improving exercise capacity.29,30 Also, sometimes there is a limitation in HFpEF diagnosis, although all our patients have HF symptoms, LVEF ≥50% and raised natriuretic peptides.
Previous studies showed that NYHA improvement is more likely in more symptomatic patients, those with absolute deficiency, and when the correct dose was administred.31,32 In our cohort, HFmrEF patients were more likely to improve NYHA class by at least one point and self-reporting clinical improvement than in the other groups, suggesting that HFmrEF and HFrEF patients behave more similarly in this aspect than HFpEF. Interestingly, this is the only group not reducing NTproBNP levels, probably due to the small number of patients in this group. Other studies, including HF patients with LVEF >40%, had not seen this NTproBNP improvement with FCM.26 The reduction of NTproBNP could be related to improving cardiomyocyte function after iron replacement, with an enhancement of diastolic function and, as previously reported in other studies, a potential improvement of ventricular function in those with LVEF >50%.33
Identifying and conveniently correcting ID is mandatory in HF patients to improve functional class and reduce HF hospitalisation with a good security profile. Despite clinical evidence, especially in HFrEF, only 27% of patients are checked to ID, and only 25% receive FCM.19 Also, it is not unusual for those with ID diagnosis to use lower doses due to logistical and economic reasons, with the consequent necessity of new infusions during the follow-up.31,32 In our study, the correct doses occur in only half of the patients, especially in HFpEF (33.7%), which means not properly replacing iron in the myocardium and probably, not improving diastolic function and functional class. Transferrin saturation seems the most potent parameter to detect and classify ID in all-range HF patients, considering that ferritin levels can be interfered with by inflammation and concomitant comorbidities, such as infection or cancer. In the CARENFER study, the authors confirmed that transferrin saturation is the more powerful parameter to detect ID, especially in the acute setting.20
LimitationsOur study had some limitations. First, there were some missing visits or incomplete data collection during follow-up, linked to the design of a retrospective study. Secondly, we did not analyse the influence of treatment optimisation on functional outcomes or the influence of other comorbidities (pulmonary disease, fragility, depression, etc.) in symptom change.
Thirdly, we have no data about hospital readmissions during the follow-up. Finally, the number of patients in HFmrEF groups is smaller than in the others, which can explain some results, although this is one of the few studies explicitly considering these patients.
ConclusionsIn conclusion, ID is frequent in HF patients regardless of LVEF and should be checked periodically. Correcting ID with FCM is a safe and effective option in all patients, improving ferric parameters. NTproBNP was reduced in HFrEF and HFpEF patients, and NYHA class improvement is less likely in HFpEF patients than in the rest of the groups.
Contribution to the fieldID is frequent in patients with HF, but the evidence in patients with HFmrEF and HFpEF is poor. In our study, the administration of intravenous FCM was safe and effective in correcting ID in HF patients regardless of LVEF at three months follow-up. The clinical benefits observed were the improvement in functional class in HFmrEF and HFrEF patients and the reduction in NT-proBNP in HFrEF and HFpEF patients. This data supports the evidence for treating ID in all HF patients with FCM.
- -
Iron deficiency is associated with exercise impairment, worse quality of life, and heart failure hospitalization.
- -
Ferric carboxymaltose is valuable and safe for correcting iron deficiency in heart failure patients.
- -
The studies with ferric carboxymaltose in patients with left ventricular ejection fraction <50% demonstrated a clinical benefit of iron deficiency correction.
- -
The usefulness of ferric carboxymaltose to correct iron deficiency would be extended to all-range left ventricular ejection fraction in heart failure patients.
- -
The use of ferric carboxymaltose is safe in heart failure patients, with a low rate of adverse effects.
- -
Iron deficiency treatment help to improve functional status and reduce natriuretic peptides in heart failure patients.
Vifor Pharma had an economic contribution to statistical analysis performance.
Authors’ contributionsStudy design: A. Esteban-Fernández, R. Bover. Data collection: A. Esteban-Fernández, M. Pérez-Serrano, R. Bover. Data review and statistical analysis: A. Esteban-Fernández, R. Bover. Preparation of the manuscript: A. Esteban-Fernández. Review, edit, and acceptance of the manuscript: all authors.
Conflicts of interestA. Esteban-Fernández and R. Bover received payments for scientific conferences from Vifor Pharma. The rest of the authors indicate no conflicts of interest.