The unique pathophysiology of COVID-19 and the complex interactions between the heart, the lungs and the inflammatory response have led some experts to propose the concept of biochemical heart failure (HF).1 To further assess this concept, we designed a dedicated substudy of the large CARD-COVID registry in order to investigate long-term clinical outcomes and quality of life among COVID-19 patients with either clinical HF or isolated elevated NT-proBNP during the index admission.
The original CARD-COVID cohort included 3080 patients with SARS-CoV-2 infection attended between March 1 and April 20, 2020. Details regarding event adjudication and short-term outcomes have already been reported elsewhere.2,3 One year after the index admission, electronic health records were reviewed, and clinical data were collected using a standardized electronic data collection form. Patients who remained alive underwent a standardized telephonic interview focused on clinical outcomes and the assessment of HF-specific health status including New York Heart Association (NYHA) class and the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12).4 Incident HF episodes and all-cause mortality during follow-up were assessed using Kaplan–Meier analysis and the log-rank test. Cox regression analysis, adjusted for age and history of chronic HF as relevant covariates, was used to assess the relationship between new HF decompensations in patients surviving the index episode and relevant baseline comorbidities, complications, and biomarker results.
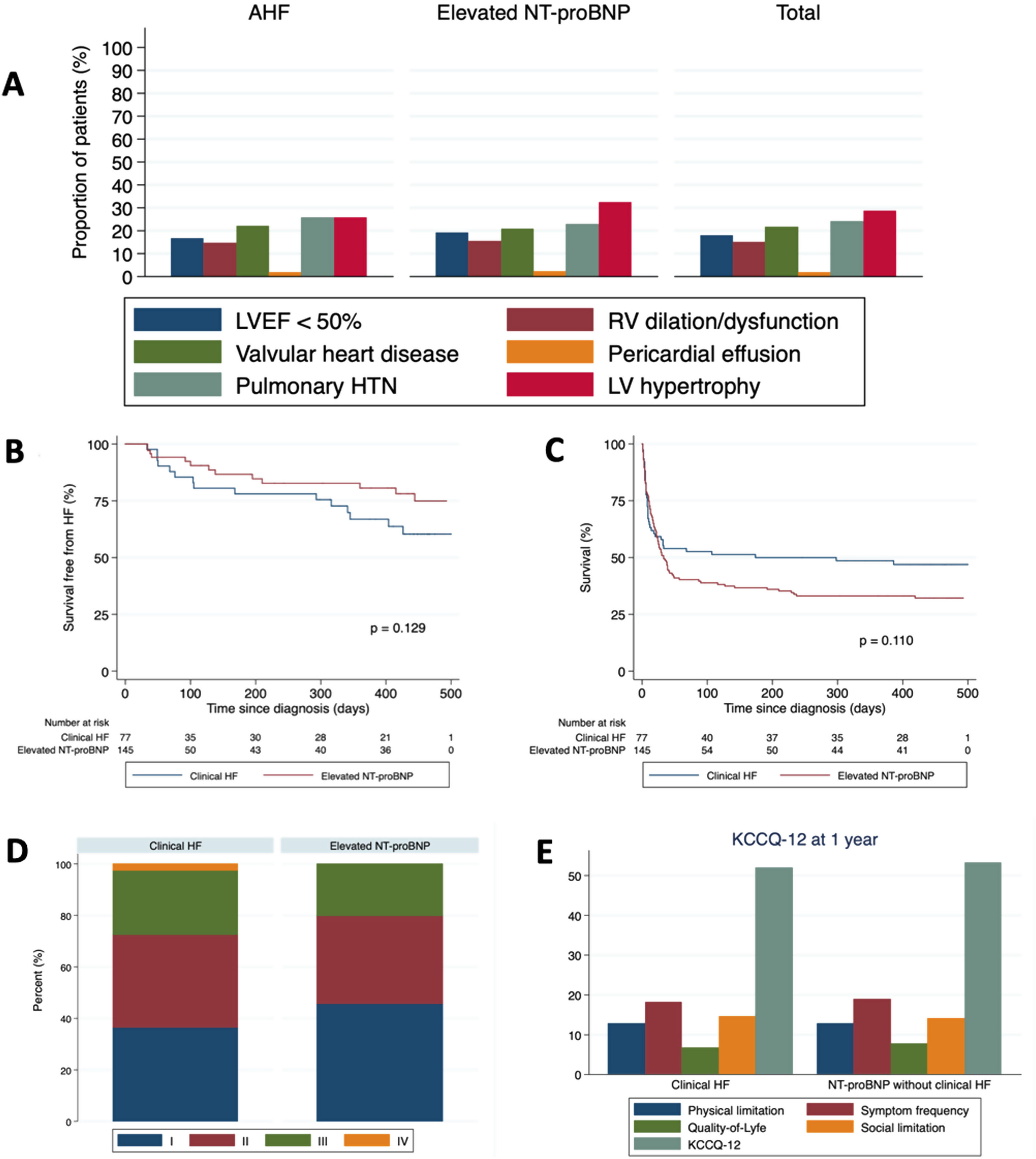
All patients were screened for participation. A total of 77 (mean age 78.6±12.6 years, 54.6% male) developed clinical HF and 145 (mean age 73.4±13.9 years, 66.2% male) showed elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) during index hospitalization and were ultimately included in the present analysis (Table 1). Reports of transthoracic echocardiograms were available for 108 patients (Fig. 1). Most clinical events concentrated during the first weeks after the diagnosis of SARS-CoV-2 infection. Median follow-up time for the 102 patients surviving the index episode was 440 (328–467) days, and 18 (17.6%) died during the study period. Importantly, there was a high incidence (27.5%) of new HF episodes in the year following SARS-CoV-2 infection, both in the clinical and in the NT-proBNP groups, and most episodes ultimately required hospital admission. Fourteen of the 28 patients with new HF episodes during follow-up (7 in the acute HF and 7 in the NT-proBNP group) had chronic HF before SARS-CoV-2 infection. Survival function analysis did not show significant differences in mortality or HF decompensation incidence between the acute HF and the isolated NT-proBNP groups (Fig. 1). Interestingly, multivariable Cox regression assessing the relationship between clinical and analytical characteristics during admission showed that inflammatory biomarkers and high sensitive-troponin I were not associated with incident HF during follow up. Only atrial arrhythmias and maximum NT-proBNP during the index admission independently predicted new HF decompensations in those patients surviving hospitalization for COVID-19 (P<.001 for both covariates). At the end of the study period, 80 of the 84 surviving patients underwent a structured telephonic interview focused on the assessment of HF-associated clinical status and quality of life (Fig. 1). Most patients were in NYHA II-IV and there were no significant differences between groups (P=.606). Interestingly, patients with either clinical HF or elevated NT-proBNP without clinical HF diagnosis showed very similar KCCQ-12 scores, both global and in each of the four clinical subdomains.
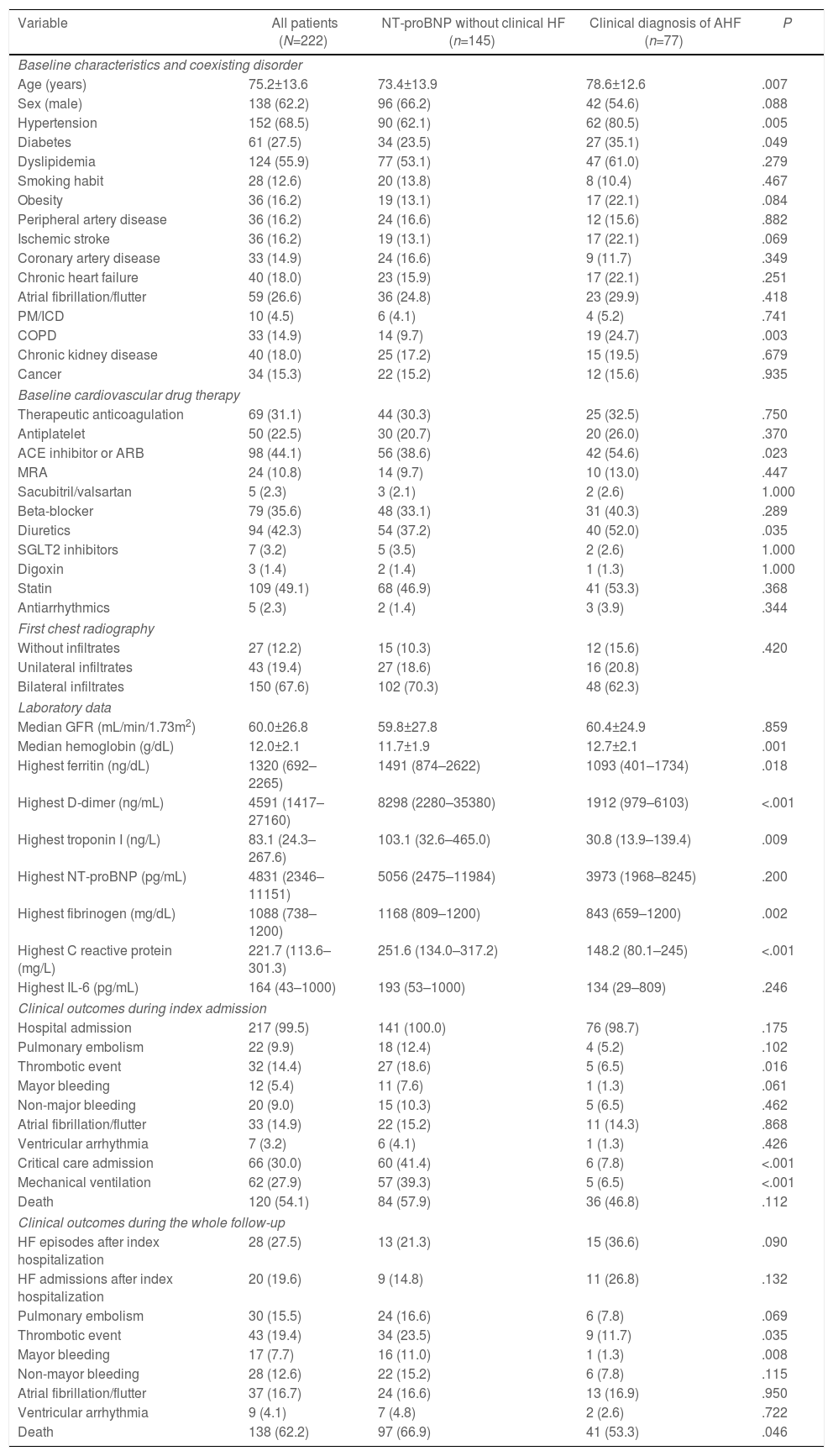
Baseline characteristics, drug therapy, vitals, and laboratory data according to clinical diagnosis of acute heart failure or isolated NT-proBNP elevation.
| Variable | All patients (N=222) | NT-proBNP without clinical HF (n=145) | Clinical diagnosis of AHF (n=77) | P |
|---|---|---|---|---|
| Baseline characteristics and coexisting disorder | ||||
| Age (years) | 75.2±13.6 | 73.4±13.9 | 78.6±12.6 | .007 |
| Sex (male) | 138 (62.2) | 96 (66.2) | 42 (54.6) | .088 |
| Hypertension | 152 (68.5) | 90 (62.1) | 62 (80.5) | .005 |
| Diabetes | 61 (27.5) | 34 (23.5) | 27 (35.1) | .049 |
| Dyslipidemia | 124 (55.9) | 77 (53.1) | 47 (61.0) | .279 |
| Smoking habit | 28 (12.6) | 20 (13.8) | 8 (10.4) | .467 |
| Obesity | 36 (16.2) | 19 (13.1) | 17 (22.1) | .084 |
| Peripheral artery disease | 36 (16.2) | 24 (16.6) | 12 (15.6) | .882 |
| Ischemic stroke | 36 (16.2) | 19 (13.1) | 17 (22.1) | .069 |
| Coronary artery disease | 33 (14.9) | 24 (16.6) | 9 (11.7) | .349 |
| Chronic heart failure | 40 (18.0) | 23 (15.9) | 17 (22.1) | .251 |
| Atrial fibrillation/flutter | 59 (26.6) | 36 (24.8) | 23 (29.9) | .418 |
| PM/ICD | 10 (4.5) | 6 (4.1) | 4 (5.2) | .741 |
| COPD | 33 (14.9) | 14 (9.7) | 19 (24.7) | .003 |
| Chronic kidney disease | 40 (18.0) | 25 (17.2) | 15 (19.5) | .679 |
| Cancer | 34 (15.3) | 22 (15.2) | 12 (15.6) | .935 |
| Baseline cardiovascular drug therapy | ||||
| Therapeutic anticoagulation | 69 (31.1) | 44 (30.3) | 25 (32.5) | .750 |
| Antiplatelet | 50 (22.5) | 30 (20.7) | 20 (26.0) | .370 |
| ACE inhibitor or ARB | 98 (44.1) | 56 (38.6) | 42 (54.6) | .023 |
| MRA | 24 (10.8) | 14 (9.7) | 10 (13.0) | .447 |
| Sacubitril/valsartan | 5 (2.3) | 3 (2.1) | 2 (2.6) | 1.000 |
| Beta-blocker | 79 (35.6) | 48 (33.1) | 31 (40.3) | .289 |
| Diuretics | 94 (42.3) | 54 (37.2) | 40 (52.0) | .035 |
| SGLT2 inhibitors | 7 (3.2) | 5 (3.5) | 2 (2.6) | 1.000 |
| Digoxin | 3 (1.4) | 2 (1.4) | 1 (1.3) | 1.000 |
| Statin | 109 (49.1) | 68 (46.9) | 41 (53.3) | .368 |
| Antiarrhythmics | 5 (2.3) | 2 (1.4) | 3 (3.9) | .344 |
| First chest radiography | ||||
| Without infiltrates | 27 (12.2) | 15 (10.3) | 12 (15.6) | .420 |
| Unilateral infiltrates | 43 (19.4) | 27 (18.6) | 16 (20.8) | |
| Bilateral infiltrates | 150 (67.6) | 102 (70.3) | 48 (62.3) | |
| Laboratory data | ||||
| Median GFR (mL/min/1.73m2) | 60.0±26.8 | 59.8±27.8 | 60.4±24.9 | .859 |
| Median hemoglobin (g/dL) | 12.0±2.1 | 11.7±1.9 | 12.7±2.1 | .001 |
| Highest ferritin (ng/dL) | 1320 (692–2265) | 1491 (874–2622) | 1093 (401–1734) | .018 |
| Highest D-dimer (ng/mL) | 4591 (1417–27160) | 8298 (2280–35380) | 1912 (979–6103) | <.001 |
| Highest troponin I (ng/L) | 83.1 (24.3–267.6) | 103.1 (32.6–465.0) | 30.8 (13.9–139.4) | .009 |
| Highest NT-proBNP (pg/mL) | 4831 (2346–11151) | 5056 (2475–11984) | 3973 (1968–8245) | .200 |
| Highest fibrinogen (mg/dL) | 1088 (738–1200) | 1168 (809–1200) | 843 (659–1200) | .002 |
| Highest C reactive protein (mg/L) | 221.7 (113.6–301.3) | 251.6 (134.0–317.2) | 148.2 (80.1–245) | <.001 |
| Highest IL-6 (pg/mL) | 164 (43–1000) | 193 (53–1000) | 134 (29–809) | .246 |
| Clinical outcomes during index admission | ||||
| Hospital admission | 217 (99.5) | 141 (100.0) | 76 (98.7) | .175 |
| Pulmonary embolism | 22 (9.9) | 18 (12.4) | 4 (5.2) | .102 |
| Thrombotic event | 32 (14.4) | 27 (18.6) | 5 (6.5) | .016 |
| Mayor bleeding | 12 (5.4) | 11 (7.6) | 1 (1.3) | .061 |
| Non-major bleeding | 20 (9.0) | 15 (10.3) | 5 (6.5) | .462 |
| Atrial fibrillation/flutter | 33 (14.9) | 22 (15.2) | 11 (14.3) | .868 |
| Ventricular arrhythmia | 7 (3.2) | 6 (4.1) | 1 (1.3) | .426 |
| Critical care admission | 66 (30.0) | 60 (41.4) | 6 (7.8) | <.001 |
| Mechanical ventilation | 62 (27.9) | 57 (39.3) | 5 (6.5) | <.001 |
| Death | 120 (54.1) | 84 (57.9) | 36 (46.8) | .112 |
| Clinical outcomes during the whole follow-up | ||||
| HF episodes after index hospitalization | 28 (27.5) | 13 (21.3) | 15 (36.6) | .090 |
| HF admissions after index hospitalization | 20 (19.6) | 9 (14.8) | 11 (26.8) | .132 |
| Pulmonary embolism | 30 (15.5) | 24 (16.6) | 6 (7.8) | .069 |
| Thrombotic event | 43 (19.4) | 34 (23.5) | 9 (11.7) | .035 |
| Mayor bleeding | 17 (7.7) | 16 (11.0) | 1 (1.3) | .008 |
| Non-mayor bleeding | 28 (12.6) | 22 (15.2) | 6 (7.8) | .115 |
| Atrial fibrillation/flutter | 37 (16.7) | 24 (16.6) | 13 (16.9) | .950 |
| Ventricular arrhythmia | 9 (4.1) | 7 (4.8) | 2 (2.6) | .722 |
| Death | 138 (62.2) | 97 (66.9) | 41 (53.3) | .046 |
ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PM, pacemaker; SGLT2, sodium-glucose cotransporter 2.
Data are expressed as no. (%) for categorical data or mean±standard deviation for continuous data.
(A) Bar plot showing the distribution of echocardiographic findings among the study patients. Valvular disease refers to moderate or severe regurgitation or stenosis according to standard definitions. Regarding RV dilation/dysfunction, pericardial effusion, RV hypertrophy and pulmonary hypertension, even mild degrees according to European Association of Cardiovascular Imaging quantification guidelines were considered. (B) Kaplan–Meier survival curves regarding freedom from HF episodes after hospital discharge. (C) Kaplan–Meier survival curves regarding all-cause mortality during the whole follow-up. (D) Proportion of patients in each New York Heart Association category in both study groups. (E) Global and domain-specific KCCQ-12 scores in both study groups. HF, heart failure; HTN, hypertension; KCCQ-12, 12-item Kansas City Cardiomyopathy Questionnaire; LV, left ventricle; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RV, right ventricle.
Even though a significant proportion of patients with clinical HF or elevated NT-proBNP died during the index hospitalization for COVID-19, our study shows that survivors still face a great burden of cardiac events, mortality, and impaired quality of life. Cardiac involvement in COVID-19 may be mediated by multiple pathways, but some researchers have focused on the identification of cardiac inflammation and myocardial injury. There is a rationale that these changes may lead to cardiac fibrosis and subsequent HF.5 However, we did not find any significant relationship between inflammatory biomarkers and high-sensitivity cardiac troponin during index hospitalization and recurrent HF episodes after hospital discharge. Elevated natriuretic peptides, despite being a reasonable surrogate of intracardiac pressures and volumes, are not 100% specific of HF, however, their plasma concentration is associated both with the likelihood of HF diagnosis and the incidence of HF episodes.6 Thus, it is interesting that we did not find significant differences between patients with a diagnosis of clinical HF and those with isolated NT-proBNP elevation during the index COVID-19 episode regarding long-term hard clinical endpoints and quality of life as assessed by the KCCQ-12.
Several limitations should be noted. The relatively small sample size may have resulted in a reduced sensitivity to detect relevant associations regarding clinical outcomes. Moreover, medical treatment at hospital discharge was not included in the original database and was not considered in the analysis. On the other hand, the incidence of thromboembolic events and development of pulmonary hypertension during follow-up was not prospectively assessed in the study cohort.
In conclusion, it may be somehow preliminary to support the concept of biochemical HF in this context,1 but our findings indeed suggest that this definition warrants further study. Besides, we hypothesize that the assessment of NT-proBNP during hospitalizations for COVID-19 may improve long-term risk stratification to guide the selection of those patients that would benefit from extended cardiovascular monitoring during follow-up.
FundingNone.
Authors’ contributionsAll the authors have made a significant contribution to the preparation of this manuscript, both in its writing (J. Caro-Codón, J.R. Rey) and in its design (J. Caro-Codón, J.R. Rey, J.L. Merino), execution (J. Caro-Codón, A. Severo Sánchez, B. Rivero Santana), data collection (J. Caro-Codón, A. Severo Sánchez, B. Rivero Santana, A. Buño) or data analysis (J. Caro-Codón, J.R. Rey, A. Buño, J.L. Merino).
Conflicts of interestNone declared.