The use of sacubitril/valsartan (SV) has shown a reduction in mortality compared to enalapril, in addition to achieving an improvement in left ventricular remodeling. There is ample evidence of its use in real life. However, there are no clinical trials that show a reduction in the indication for implantable cardioverter defibrillator (ICD) after its use.
MethodsProspective real-life study of patients with heart failure and reduced ejection fraction treated with SV, starting in the outpatient phase or during hospitalization. An analysis of individual and combined endpoints for efficacy and safety is carried out, including the change of indication of ICD with the use of SV.
ResultsAfter 12 months, a clear reduction in the indication for ICD was achieved, an improvement in left ventricular remodeling, a reduction in natriuretic peptides, and a reduction in readmissions for heart failure. In addition, showing clear safety and a low rate of drug discontinuation (6%).
ConclusionsThe results of this real-life study achieved a combined endpoint of efficacy in 88.3% of patients, demonstrating the safety of SV. On the other hand, the indication for ICD is drastically reduced, so the moment to indicate an ICD should be clarified through clinical trials.
El uso del sacubitrilo-valsartán (SV) ha mostrado una reducción de la mortalidad frente a enalapril, además de lograr mejoría del remodelado del ventrículo izquierdo. Existe una amplia evidencia de su uso en vida real. Sin embargo, no existen ensayos clínicos que muestren la reducción de indicación de desfibrilador automático implantable (DAI) tras su uso.
MétodosEstudio prospectivo en vida real de pacientes con insuficiencia cardiaca y fracción de eyección reducida tratados con SV, con inicio en fase ambulatoria o durante hospitalización. Se lleva a cabo un análisis de endpoints individuales y combinados de eficacia y seguridad, incluido el cambio de indicación de DAI con el uso del SV.
ResultadosTras 12 meses de uso del SV, se logró una clara reducción de indicación de DAI, mejoría del remodelado del ventrículo izquierdo, reducción de los péptidos natriuréticos y disminución de los reingresos hospitalarios por insuficiencia cardiaca. Además, se mostró una clara seguridad y baja tasa de discontinuación del fármaco (6%).
ConclusionesLos resultados de este estudio en vida real lograron un compuesto de eficacia en el 88,3% de los pacientes y demostraron la seguridad del SV. Por otro lado, se redujo de forma drástica la indicación de DAI, por lo que debería aclararse mediante ensayos clínicos el momento de indicar un DAI.
Heart failure (HF) affects more than 23 million people around the world,1 it represents one of the main causes of cardiovascular morbidity and mortality, its prevalence increasing with age.2 The latest clinical practice guidelines3 classify HF into three groups according to left ventricular ejection fraction (LVEF): HF with reduced LVEF (< 40%), mid-range LVEF (40–49%), and preserved LVEF (≥ 50%), resulting in different repercussions on therapeutic patient management.
Heart failure and reduced ejection fraction (HFrEF) treatment comprises neurohormonal antagonists, including angiotensin converting enzyme inhibitors or angiotensinogen receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists, which have been shown to improve prognosis. Recently, sacubitril/valsartan (SV) has joined this therapeutic arsenal. This drug combines a neprilysin inhibitor and angiotensin II receptor antagonist, resulting in an increase in natriuretic peptide levels, while inhibiting the effects of angiotensin II. SV has been shown to be superior to enalapril in reducing the risk of death and hospitalization for HF.4 The combination with the best efficacy on patient prognosis has proven to be angiotensin II receptor antagonist+beta-blockers+mineralocorticoid receptor antagonists.5
The SV efficacy has been demonstrated in several randomized clinical trials that compared this combination to enalapril. The results of the PARADIGM-HF,4 an international double-blind clinical trial in patients with chronic HF that were previously treated using angiotensin-converting enzyme inhibitors and beta-blockers,4 have revealed that SV significantly improves the prognosis of HFrEF patients in New York Heart Association (NYHA) class II–IV, as compared to enalapril. In addition, SV was demonstrated to reduce long-term mortality, both overall and of cardiovascular origin, and the need of hospital readmission due to worsening HF. On the other hand, data from the PIONEER-HF clinical trial6 showed that, in patients admitted for acute HF, SV similarly reduces N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels and readmission rates compared to enalapril.
Data from the PROVE-HF clinical trial7 have shown that there was a correlation between the reduction in NT-proBNP levels after SV therapy in HFrEF patients and several signs of reverse cardiac remodeling at one-year follow-up. Recently a subanalysis of the PROVE-HF have shown that among a cohort of patients with HFrEF who met primary prevention implantable cardioverter defibrillator (ICD) eligibility criteria at baseline, 32% improved their ejection fraction to >35% by 6 months and 62% to >35% by 12 months after initiation of SV therapy, with relevant clinical implications.8
Based on the drug's prescribing information,9 SV administration is allowed if systolic blood pressure≥100mmHg, potassium level<5.4mmol/L, and estimated glomerular filtration rate>60mL/min/1.73m2. Caution is warranted in the event of estimated glomerular filtration rate of 30–60mL/min/1.73m2, whereas SV is definitely discouraged in patients with end-stage renal disease. These criteria are in line with those of the main trial proving the drug's efficacy and safety.5 A few evidences have been reported in the literature supporting its use outside of these criteria.10
The use of an ICD to avoid sudden cardiac death is indicated in patients with symptomatic HF (NYHA Class II/III) and LVEF≤35% after ≥3 months of optimal medical therapy. SV treatment in patients with LVEF≤35% has been associated with LVEF recovery and improvement in functional class.4,6,7 This implies that a few patients that, prior to SV therapy, displayed an indication for an ICD based on the current guidelines3 would no longer require such an ICD intervention. This paradigm opens an entirely new approach to preventn sudden death in HFrEF patients.
This study sought to analyze SV effectiveness and safety in HFrEF patients in real-world clinical practice and evaluate its impact on other outcome measures, including the indication for ICD.
MethodsWe performed a prospective study in real-world SV-treated patients with HFrEF. The study was carried out in one single center between 2017 and 2019 (Hospital Universitario Juan Ramón Jiménez, a tertiary hospital). This center's HF unit comprises three cardiologists and two nurses specialized in HF care HF unit is considered the reference unit for a population of 550000 people, with around 800 patients admitted year.
Ethical approval for the study was obtained from the hospital's Clinical Investigation Ethics Committee (Fundación Andaluza Beturia para la Investigación en Salud) and informed consent was obtained from the patients in writing.
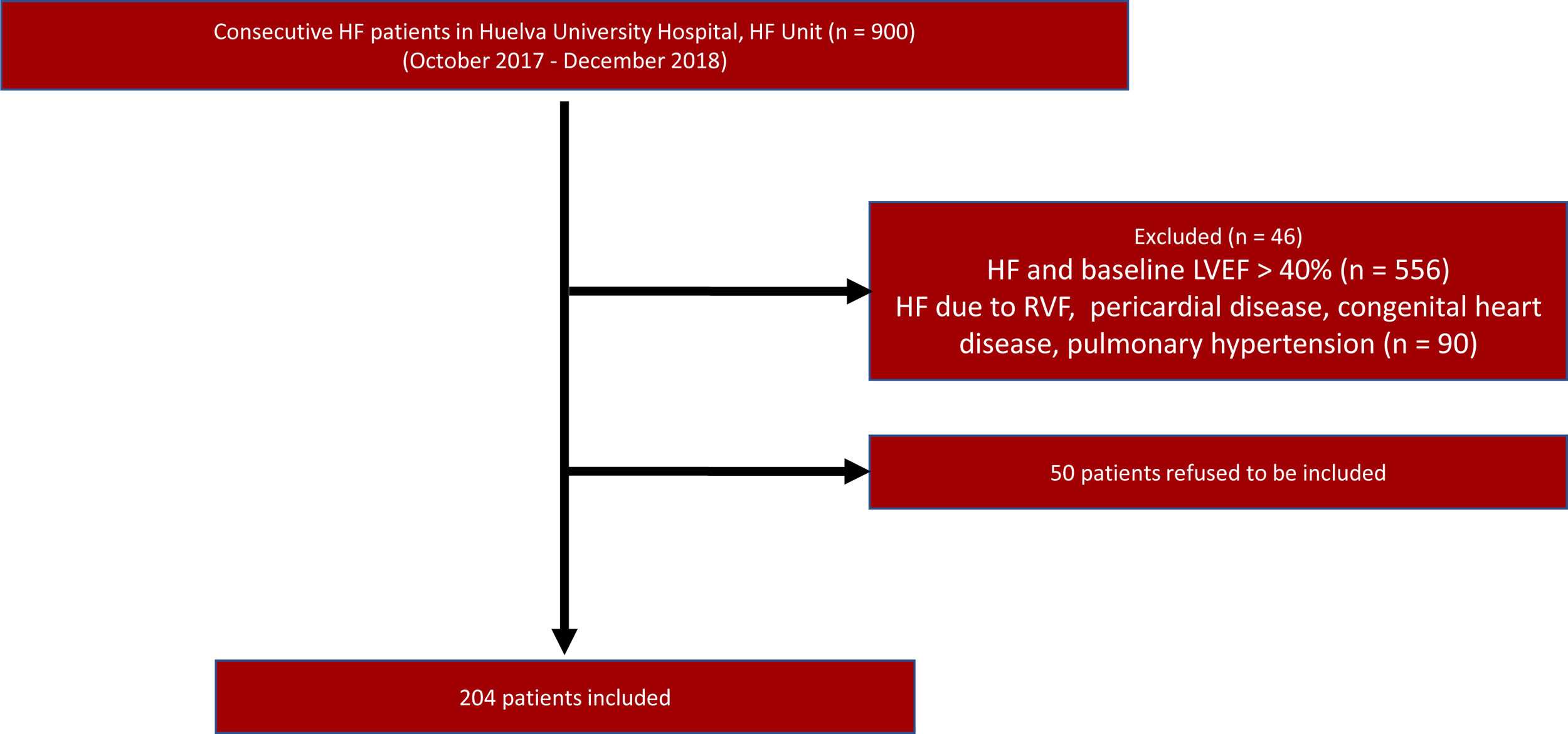
PatientsAll consecutive HFrEF patients attending the Heart Failure Unit of the Juan Ramon Jimenez University Hospital who started SV treatment between October 2017 and December 2018 were included (N=204), regardless of whether they were in or outpatients. Fig. 1 shows patients enrolment into the study. HF definition of HF was consistent with that of the European Society of Cardiology guidelines,3 including presentation of typical HF symptoms that may be accompanied by HF signs caused by a structural of functional cardiac abnormalities. HFrEF is defined by the presence of HF symptoms in a NYHA Class II, III, or IV patient, with LVEF<40%. The inclusion criteria for the study were: (a) adult patients (18 years old and over) that fulfilled the HF diagnosis; (b) LVEF<40% confirmed by echocardiography. The exclusion criteria were: (a) patients with LVEF≥40%; (b) concomitant dementia or other conditions with a life-expectancy of < 1 year; (c) potential follow-up difficulties.
Variables and measurementsDemographic data, baseline characteristics, medical history including prior treatments, NYHA functional class, LVEF, vital signs, and laboratory parameters before starting SV treatment were collected. The follow-up period was 12 months. Patients were evaluated within the first month after starting SV treatment depending on their individual needs, and then every three months until the end of the 12-month follow-up. The protocol for the follow-up visits comprised a medical examination, anamnesis of relevant clinical features, signs, and symptoms, medication, NYHA functional class, SV de-escalation or withdrawal, potential undesirable effects attributable to SV therapy (symptomatic hypotension, impaired renal function, hyperkalemia, or angioedema), blood testing, electrocardiogram, and treatment adjustment of treatments as necessary according to the clinical practice guidelines. An echocardiography was performed at 12 months or earlier in the case of worsening HF at the discretion of cardiologist.
Clinical outcomesThe study outcomes were effectiveness and safety, measured by individual parameters and combined endpoints, with pre- and post-practice data compared.
The effectiveness parameters applied were LVEF, NT-proBNP levels, indication for ICD, functional class, and number of hospitalizations. In addition, there was a combined endpoint including any of the following three criteria: increase in LVEF, reduction in hospital admissions, or improvement in functional class.
Regarding safety, data on creatinine, potassium, blood pressure, percentage of patients hat attained the maximum dose, and percentage of patients that discontinued treatment were recorded. The combined safety endpoint was defined by the absence of any of the following three criteria: hyperkalemia>5.5; creatinine>2.5, and symptomatic hypotension.
Statistical analysesA descriptive analysis was carried out using central tendency and dispersion measures, as well as frequency and percentage distribution for quantitative and qualitative variables, respectively. Possible differences in baseline characteristics between our cohort and that of the PARADIGM-HF5 clinical trial were analyzed using parametric or non-parametric tests depending upon the distribution of quantitative variables, and differences of proportions and Chi-squared test for the qualitative ones.
The comparison of individual effectiveness and safety parameters from baseline and 12 months after treatment initiation was carried out using tests for paired data like the paired Student's t-test and McNemar's test.
The association of effectiveness and safety with different subgroups was studied, using the odds ratio (OR) as an association measure. These analyzes were performed in the following subgroups: inpatient or outpatient; estimated glomerular filtration rate<30mL/>30mL/min/1.73m2; systolic blood pressure≤100mmHg/>100mmHg; NYHA II vs NYHA III/IV. Statistical analysis was carried out using STATA Version 12 (Stata Corporation, College Station, United States).
ResultsStudy cohortThe study cohort included 204 patients, who were mostly male (78%), with a mean age of 66±11 years, median HF duration of 2.1 years, and average LVEF of 29.8±6.3%. The most common etiology was ischemic (54%), followed by idiopathic (26%). The distribution of patients by functional NYHA class was: 63.3% Class II, 29.1% Class III, 6.5% Class I, and 1% Class IV. Median values (P25–P75) for NT-proBNP and systolic blood pressure levels were 1803pg/mL (873–3864pg/mL) and 120mmHg (110–135mmHg), respectively, with mean values (± standard deviation [SD]) for serum creatinine of 1.33±1.45mg/dL. Concerning comorbidity, 47% of patients exhibited prior acute myocardial infarction, 71% hypertension, 39% diabetes, 65% dyslipidemia, and 38% atrial fibrillation (Table 1).
Study cohort: baseline characteristics.
| Variable | N=204 | Value |
|---|---|---|
| Gender (male),n(%) | 160 (78.4%) | |
| Age (years), mean±SD | 66.0±11.2 | |
| Duration of heart failure, median (P25–P75) | 2.1 (0.5– 6.7) | |
| Number of hospitalizations, median (P25–P75) | 0 (0–1) | |
| Heart rate, mean±SD | 65.8±13.5 | |
| SBP (mm Hg), median (P25–P75) | 120 (110–135) | |
| DBP (mm Hg), median (P25–P75) | 70 (60–80) | |
| Comorbidities | ||
| Hypertension, n (%) | 144 (70.9) | |
| Hypercholesterolemia, n (%) | 132 (65.0) | |
| Diabetes, n (%) | 80 (39.2) | |
| Anemia, n (%) | 22 (10.8) | |
| Previous atrial fibrillation, n (%) | 77 (37.8) | |
| Previous percutaneous transluminal angioplasty, n (%) | 96 (47.0) | |
| Smoking, n (%) | ||
| Ex-smoker | 126 (61.8) | |
| Current smoker | 18 (8.8) | |
| Ictus, n (%) | 22 (10.8) | |
| Obesity, n (%) | 42 (20.6) | |
| Chronic obstructive pulmonary disease, n (%) | 42 (20.6) | |
| Obstructive sleep apnea, n (%) | 11 (5.4) | |
| Previous myocardial infarction, n (%) | 97 (47.6) | |
| Peripheral artery disease, n (%) | 15 (7.4) | |
| Dementia, n (%) | 1 (0.5) | |
| Valvular prosthesis, n (%) | 14 (6.9) | |
| Cardiomyopathy | ||
| Ischemic, n (%) | 111 (54.4) | |
| Non-ischemic, n (%) | 90 (44.1) | |
| Hypertensive, n (%) | 4 (4.4) | |
| Tachycardia-induced, n (%) | 6 (6.7) | |
| Valvular, n (%) | 8 (8.9) | |
| Familiar, n (%) | 6 (6.7) | |
| Idiopathic, n (%) | 23 (25.6) | |
| Myocarditis, n (%) | 2 (2.2) | |
| LVEF (%), mean±SD | 29.8±6.3 | |
| ACEI/ARB previous (%) | 96.9 | |
| Beta-blockers (%) | 94.7 | |
| MRA (%) | 81.6 | |
| Ivabradine (%) | 37.5 | |
ACEI, angiotensin-converting-enzyme inhibitors; ARB, angiotensin receptor blockers; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure; SD, standard deviation.
The comparison with the PARADIGM data trial5 showed that our study cohort patients were older and displayed worse kidney function, higher natriuretic peptides levels, and worse functional class (Table 2).
Comparison of baseline characteristics versus PARADIGM-HF.
| Variable | Our sample | PARADIGM | P |
|---|---|---|---|
| (n=204) | (n=8442) | ||
| Gender (female), n (%) | 166 (21.6%) | 1857 (22.0%) | 1.000 |
| Age (years), mean±SD | 66.0±11.2 | 63.8±11.5 | .007 |
| Heart rate, mean±SD | 65.8±13.5 | 74±12 | <.0001 |
| SBP (mm Hg), mean±SD | 123±21 | 121±15 | .063 |
| NT-proBNP (pg/mL), median (P25–P75) | 1803 (873–3864) | 1631 (885–3154) | – |
| Creatinine (mg/dL), mean±SD | 1.33±1.45 | 1.13±0.3 | <.0001 |
| Hypertension, n (%) | 144 (70.9%) | 5985 (70.9%) | 1.000 |
| Diabetes, n (%) | 80 (39.2%) | 2929 (34.7%) | .237 |
| Previous myocardial infarction, n (%) | 97 (47.6%) | 5065 (59.9%) | .001 |
| LVEF (%), mean±SD | 29.8±6.3 | 29.6±6.1 | .644 |
| NYHA functional class, n (%) | |||
| I | 13 (6.5%) | 422 (5.1%) | |
| II | 126 (63.3%) | 5909 (70.2%) | |
| III | 58 (29.1%) | 2026 (24.0%) | |
| IV | 2 (1.0%) | 68 (0.8%) | |
| NYHA functional class, n (%) | .088 | ||
| I–II | 139 (69.8%) | 6331 (75.1%) | |
| III–IV | 60 (30.2%) | 2094 (24.9%) | |
LVEF, left ventricle ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
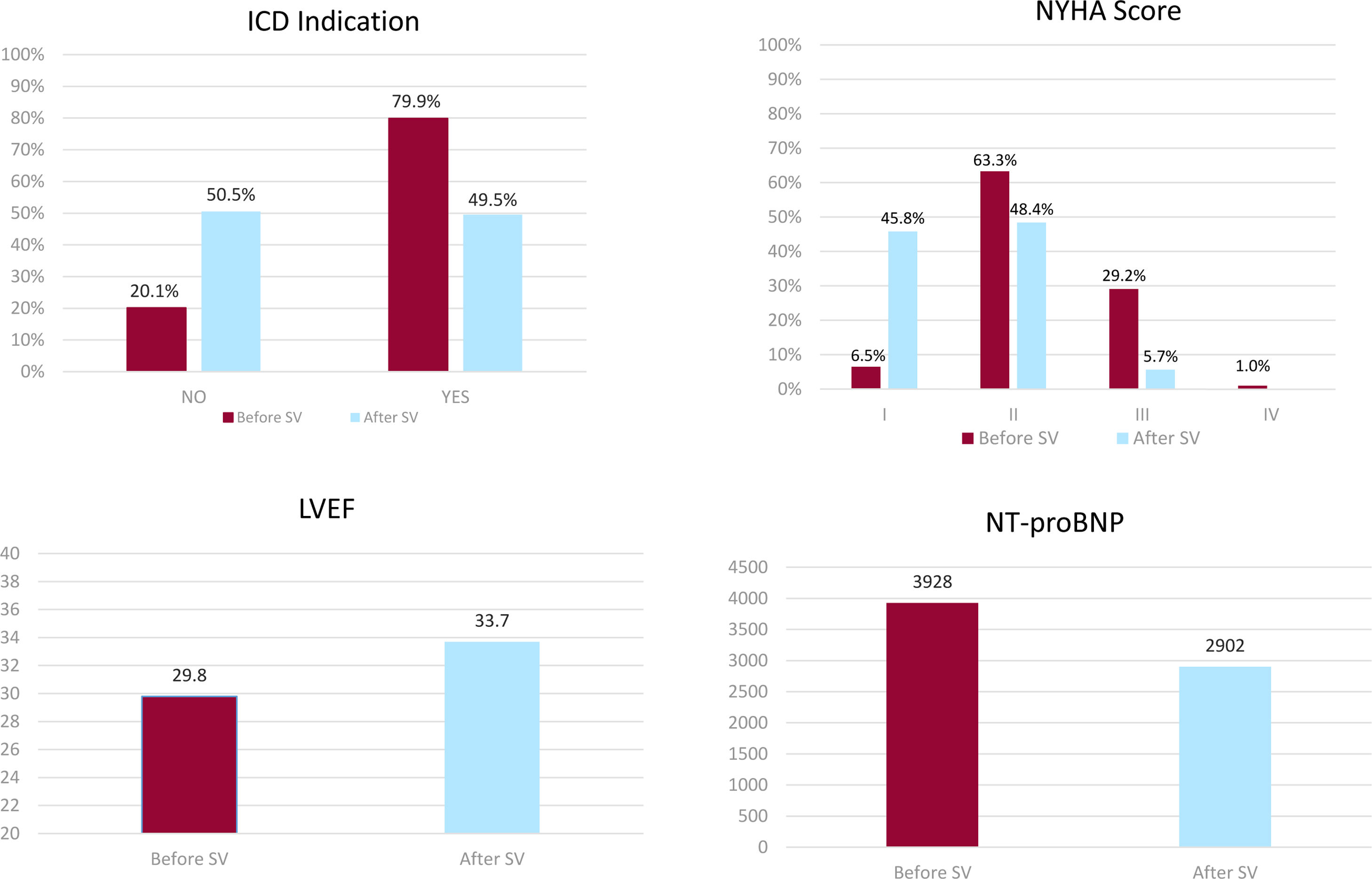
Comparative efficacy parameters recorded before and after SV treatment have been illustrated in Fig. 2. This illustration reveals a significant increase in LVEF (29.8% vs 33.7%; P<.0001), along with a marked decrease in patients with ICD indication (79.9% vs 49.5%; P<.0001), an important decrease in NT-proBNP levels (3928 vs 2902pg/mL; P=.012), and a strong reduction in hospital admissions (141 vs 35; P<.0001). In addition, SV was shown to significantly improve patients’ functional status, so that the percentage of Class I patients changed from 6% to 46%; Class II from 63% to 48%; Class III from 29% to 6% (Table 3).
Efficacy: before-after sacubitril/valsartan and combined endpoint.
| Individual efficacy parameter | N | Baseline | After SV | P |
|---|---|---|---|---|
| LVEF (%), mean±SD | 191 | 29.8 (6.4) | 33.7 (8.0) | <.0001 |
| NT-proBNP (pg/mL), mean±SD | 196 | 3928 (6594) | 2902 (6141) | .012 |
| HF admission, n | 203 | 141 | 35 | <.0001 |
| Loop diuretic (mg), mean±SD | 152 | 39.8 (31.5) | 39.9 (36.1) | .947 |
| ICD indication, n (%) | 204 | 163 (79.9%) | 101 (49.5%) | <.0001 |
| NYHA functional class, n (%) | ||||
| I | 13 (6.5%) | 88 (45.8%) | <.0001 | |
| II | 126 (63.3%) | 93 (48.4%) | ||
| III | 58 (29.1%) | 11 (5.7%) | ||
| IV | 2 (1.0%) | – | ||
HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
The combined efficacy endpoint was defined by the presence of any of the following three criteria: increase in LVEF, reduction in hospital admissions, or improvement in functional class. The number of patients meeting any of these criteria was 84 (41%) for reduction in hospitalizations, 114 (60.6%) for improvement in functional class; 91 (56.5%) for improvement in LVEF. A total of 166 cohort patients (81.3%) met the combined efficacy endpoint.
Patients who met this combined endpoint had lower LVEF at diagnosis (29.6 vs 32.5; P=.040), worse functional class (P=.001), higher number of hospitalizations (P=.009), higher frequency of diabetes (43% vs 18%; P=.035), better renal function with lower creatinine levels (2.13 vs 1.25; P=.009), and higher rate of glomerular filtration (72.2 vs 59.5; P=.028) (Table 4).
Efficacy with sacubitril/valsartan concerning achievement of combined endpoint.
| Basal characteristics | Combined efficacy endpoint | P | |
|---|---|---|---|
| No (N=22) | Yes (N=166) | ||
| Place where the treatment was started | .744 | ||
| Inpatients | 20 (90.9%) | 143 (86.1%) | |
| Outpatients | 2 (9.1%) | 23 (13.9%) | |
| Age (years) | 67.0±13.9 | 66.0±10.9 | .687 |
| Gender (male), n (%) | 18 (81.8%) | 128 (77.1%) | .788 |
| SBP, mean±SD | 120.7±16.6 | 122.8±21.3 | .660 |
| Diabetes | 4 (18.2%) | 72 (43.4%) | .035 |
| Atrial fibrillation | 12 (54.5%) | 57 (34.3%) | .065 |
| Heart Failure duration (years), mean±SD | 6.2±6.1 | 4.2±5.0 | .087 |
| Ischemic, n (%) | 11 (50.0%) | 90 (54.2%) | .709 |
| LVEF at diagnosis (%), mean±SD | 32.5±5.6 | 29.6±6.3 | .040 |
| QRS duration (ms), mean±SD | 131.5±32.4 | 122.7±23.7 | .221 |
| NT-proBNP (pg/mL), mean±SD | 1738±1506 | 4368±7065 | .100 |
| NYHA functional class, n (%) | |||
| I | 1 (4.5%) | 7 (4.3%) | |
| II | 21 (95.4%) | 98 (60.5%) | .001 |
| III | – | 55 (33.9%) | |
| IV | – | 2 (1.2%) | |
| N° of admissions | 0.27±0.55 | 0.82±0.95 | .009 |
| Creatinine (mg/dL), median (P25–P75) | 2.13±2.95 | 1.25±0.09 | .009 |
| eGFR (CKD-EPI) (mL/min/1.73m2), median (P25–P75) | 59.5±27.5 | 72.2±25.2 | .028 |
| Loop diuretics, n (%) | 15 (68.2%) | 106 (64.2%) | .716 |
| Angiotensin-converting-enzyme inhibitors, n (%) | 20 (91%) | 154 (93.3%) | .367 |
| Betablockers, n (%) | 22 (100%) | 154 (93.3%) | .367 |
| Ivabradine, n (%) | 5 (22.7%) | 52 (31.5%) | .400 |
| Mineralcorticoid receptors antagonists, n (%) | 17 (77.3%) | 136 (82.4%) | .556 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
Finally, subgroup analysis revealed that efficacy was significantly higher in patients who met the PARADIGM trial5 criteria (OR=2.76; P=.046), with decreased efficacy observed when basal glomerular filtration was < 30 (OR=0.15; P=.003).
Outcomes: safetyComparison of safety parameters recorded at baseline and at 12-month follow-up showed no significant difference in serum creatinine, potassium, and systolic blood pressure values. It should be noted that only 6% of patients discontinued treatment, and that 38% attained a final dose of 97/103mg (Table 5).
Safety: before–after sacubitril/valsartan and concerning achievement of combined endpoint.
| Safety | N | Before SV | After SV | P |
|---|---|---|---|---|
| Creatinine (mg/dL), mean (SD) | 181 | 1.30 (1.53) | 1.61 (2.24) | .311 |
| Potassium (mEq/L), mean (SD) | 180 | 4.7 (4.6) | 4.6 (4.5) | .208 |
| SBP (mm Hg), mean (SD) | 182 | 120.6 (17.5) | 117.8 (17.4) | .152 |
| Final dose | ||||
| Retired | 198 | 12 (6.1%) | ||
| 24/26mg | 34 (17.2%) | |||
| 49/51mg | 76 (38.4%) | |||
| 97/103mg | 76 (38.4%) | |||
| Basal characteristics | Combined safety endpoint | P | |
|---|---|---|---|
| No (N=45) | Yes (N=152) | ||
| Place where the treatment was started | .162 | ||
| Inpatients, n (%) | 36 (80.0%) | 134 (88.2%) | |
| Outpatients, n (%) | 9 (20.0%) | 18 (11.8%) | |
| Age (years), mean±SD | 65.8±9.9 | 66.1±11.4 | .871 |
| Gender (male), n (%) | 37 (82.2%) | 119 (78.3%) | .568 |
| SBP, mean±SD | 123.4±21.6 | 122.6±20.8 | .837 |
| Diabetes, n (%) | 22 (48.9%) | 56 (36.8%) | .147 |
| Atrial fibrillation, n (%) | 16 (35.6%) | 59 (38.8%) | .692 |
| HF duration (years),mean±SD | 4.4±4.7 | 4.2±5.1 | .792 |
| Ischemic, n (%) | 27 (60.0%) | 83 (54.6%) | .522 |
| LVEF (%), mean±SD | 30.2±6.5 | 29.9±6.2 | .762 |
| QRS duration (ms), mean±SD | 118.9±23.8 | 124.3±25.5 | .257 |
| NT-proBNP (pg/mL), mean±SD | 6070±10144 | 3223±4861 | .013 |
| NYHA functional class, n (%) | .024 | ||
| I | 3 (6.7%) | 10 (6.8%) | |
| II | 21 (46.7%) | 100 (68.0%) | |
| III | 21 (56.7%) | 35 (23.8%) | |
| IV | – | 2 (1.4%) | |
| N° of admissions, mean±SD | 0.96±1.10 | 0.63±0.85 | .037 |
| Creatinine (mg/dL), mean±SD | 1.70±2.11 | 1.24±1.21 | .072 |
| eGFR (CKD-EPI) (ml/min/1.73m2), mean±SD | 68.2±29.8 | 71.0±24.0 | .510 |
| Starting dosage | 1.42±0.54 | 1.55±0.62 | .203 |
| Potassium (mEq/L), mean±SD | 4.67±0.49 | 4.63±0.46 | .597 |
| Criteria for ICD before the treatment, n (%) | 33 (73.3%) | 123 (80.9%) | .271 |
| Anemia, n (%) | 7 (15.6%) | 14 (9.2%) | .226 |
| Dementia, n (%) | – | 1 (0.7%) | 1.000 |
| Angiotensin-converting-enzyme inhibitors, n (%) | 41 (93.2%) | 144 (94.7%) | .713 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
The only differences observed between patients who met and those who did not meet the combined safety criteria were the lower concentration of NT-proBNP (3223 vs 6070pg/mL; P=.013), lower functional class (P=.024), and lower number of hospitalizations (0.63 vs 0.96; P=.037) (Table 5).
Subgroup analysis revealed that SV was safer in patients with glomerular filtration above 30mL/min/1.73m2 and better functional class, with OR of 0.27 (P=.030) and 0.38 (P=.007), respectively. There were no differences between inpatient and outpatient setting, regardless of blood pressure values or whether or not PARADIGM inclusion criteria were met.
DiscussionTo our knowledge, this is one of the most comprehensive studies on SV in HFrEF patients carried out to date. The study included outpatients and inpatients, in addition to involving different efficacy and safety outcomes, some of which have not yet been studied in previously conducted clinical trials According to the results obtained, SV treatment for 12 months exerts the following effects: a) clear reduction in ICD indication; b) increase in LVEF, decrease in NT-proBNP and readmission; rates; c) combined efficacy endpoint achieved in 81.3% of patients. In addition, SV has proven to be safe without resulting in creatinine concentration, potassium level, or systolic blood pressure value changes, and with only a low discontinuation rate (6%).
Despite the observation that clinical trials are the first-choice tool for evaluating the efficacy of drugs, we should not forget that patients included in clinical trials are highly selected; generally, they do not represent real-life settings.10 This underlines the relevance of conducting real-life studies like the one reported herein in order to be enable us to assess their effectiveness in real-life patients across the entire disease spectrum. Indeed, our population also included older patients with more advanced heart disease and worse kidney function than previous SV clinical trials have done.4,6,7
Different observational studies have been carried out on the SV use under real-life conditions. Regarding efficacy endpoints, this drug has been shown to be effective in improving LVEF11–14 and functional outcome by improving NYHA class,12,13,15,16 while reducing hospital admissions for worsening HF.13,15–17 Also, a recent study focused on changes in ICD indications following SV treatment.8Table 6 provides a summary of the most relevant studies under real-life conditions.14-19
Main characteristics of real-life studies with sacubitril/valsartan.
| Author | Patients (n) | NYHA class | LVEF (%) | Final dose SV | NYHA change | Final LVEF (%) | Loss of ICD indication | HF admissions |
|---|---|---|---|---|---|---|---|---|
| Esteban A14 | 427 | II: 68%III:27% | 29 | Low 21%Mid 33%High 33% | Yes | 33 | N/A | Decrease |
| Pharithi RB15 | 322 | I: 10%II: 78%III:11% | 28 | Low 8%Mid 11%High 80% | Yes | 32 | N/A | N/A |
| González L16 | 250 | N/A | 31 | N/A | N/A | 36 | N/A | N/A |
| Lopez JC17 | 527 | II: 63%III:30% | 30 | Low 27%Mid 35%High 36% | Yes | 30 | N/A | Decrease |
| Martens P18 | 201 | II: 68%III: 31% | 29 | Low 33%Mid 42%High 25% | Yes | N/A | N/A | Decrease |
| Chang HY19 | 466 | II: 79% | 27 | N/A | N/A | N/A | N/A | Decrease |
HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; N/A, non-available; NYHA, New York Heart Association; SV, sacubitril/valsartan.
The study's efficacy results showed that 81% of SV-treated patients attained the combined efficacy endpoint (defined by at least one of the following criteria: improvement in LVEF, better functional class, or fewer hospital readmissions. It must be stressed that although various studies have published efficacy results based on different parameters, none of them have used a combined measurement as in our case, which can be considered an estimate of overall drug efficacy.
Advances made in HF management have enabled us to reduce sudden death in HFrEF patients, through implementing optimal medical therapy18 and using various devices.19 Despite these major advances made, more than one-third of all-cause mortality in contemporary clinical trials is still to be accounted for by sudden cardiac death.20 ICD implantation has proven its long-term efficacy in preventing patients from sudden cardiac death,21 but approach has a high economic cost, and it is not devoid of serious harm.22,23 The cost-effectiveness of ICD implantation for primary prevention in patients with a LVEF<40% and ischemic or non-ischemic heart disease has been previously analyzed.22 These data have revealed that the mean lifetime cost of ICD was shown to be much higher. Accordingly, the cost of a ‘no ICD strategy’ was € 50685±€ 4604 vs € 86759±€ 3343 for an ‘ICD strategy’, although the ICD implantation strategy was cost-effective. Regarding complications, van der Heijden et al.23 showed that the 12-year ICD complication rate, with or without cardiac resynchronization therapy, was 20% (95% confidence interval [95%CI], 18–22%) for inappropriate shock, 6% (95%CI, 5–8%) for device-related infection, and 17% (95%CI, 14–21%) for lead failure. These study results underline that a decrease in ICD indications likely achieves significant cost saving and an improvement in HF patients’ quality of life.
The reduction in ICD indications achieved in our study (79.9% prior to 49.5% at 12 months; P<.00001) proves to be very interesting information. In an article published by El Battrawy et al.,24 SV failed to decrease ventricular arrhythmias after 12-month follow-up, whereas in a recent article by Rohde et al.,25 it is concluded that the benefit of such a reduction is greater in ICD users and non-ischemic cardiomyopathy patients. Nevertheless, no direct comparison between ICD use and SV addition has been published so far. Yet, in some studies, SV has been shown to effectively reduce ventricular tachycardia and ventricular fibrillation rates, while decreasing the number of ICD-delivered shocks.26,27 Consequently, SV treatment may definitely turn out to be cost-effective versus ICD implantation.28
On the other hand, our data show a reverse remodeling with improvement in LVEF (from 29.8 to 33.7%; P<.0001); these results are similar to those published by Martens et al.29 The mean value of NT-proBNP was reduced (3928 to 2903pg/mL; P=.012), together with a marked improvement in functional class (40% remained asymptomatic after using SV), as described by Lau et al.30
As for the drug's safety in our sample, neither worsening of creatinine or potassium values nor changes in systolic blood pressure were observed. Not surprisingly, the drug discontinuation rate was only 6%.
LimitationsThe main limitation of this registry is its observational and not randomized design. This introduces a possible selection bias that could affect its external validity. Other limitations of the study are its small sample size and the lack of comparison between different subgroups. All this indicates that the results of this work should be considered simply as generators of hypotheses and that randomized studies are necessary to confirm our findings.
ConclusionsThe results of this real-life study prove that SV treatment attains effectiveness in 88.3% of patients without any associated safety concerns. In addition, the improvement in left ventricle remodeling and reduction in ICD indications observed are likely to significantly impact patients’ prognosis and quality of life, all of which at a lower cost.
- •
SV has demonstrated to reduce long-term mortality in HfrEF, as compared to enalapril.
- •
SV has shown signs of reverse cardiac remodeling.
- •
ICD in primary prevention decreases mortality in HFrEF.
- •
The indication for ICD implantation is reduced by 30.4% with the use of SV.
- •
Effectiveness and safety demonstrated in an unselected and underrepresented population in clinical trials.
This work was not funded.
Authors’ contributionsAll the authors have participated in the writing of the article and have accepted the conditions for its publication.
Conflicts of interestNone.
Abbreviations: HF: heart failure; HFrEF: heart failure and reduced ejection fraction; SV: sacubitril/valsartan; NT-proBNP: N-terminal pro-B-type natriuretic peptide.