Since its introduction in 2002, the number of transcatheter aortic valve implantation (TAVI) procedures has grown exponentially. Although technical advances have been made in valve technology and its implantation system, conduction disturbances (CD) remain the most common complication of the procedure. CD have a negative impact on the prognosis of patients for reasons such as the worsening of left ventricular ejection fraction, the need for a pacemaker or the increased risk of sudden cardiac death. Several factors have been associated with higher risk of CD, including prior right bundle-branch block, valve type and deep valve placement inside the left ventricular outflow tract.
Whilst CD are the most common complications of TAVI, there is still no general agreement on the optimal strategy to minimize its incidence. In this review we will approach the current evidence for the pathophysiology, incidence, clinical implications and management of CD in TAVI.
Desde su introducción en 2002, el número de implantes percutáneos de válvula aórtica (TAVI) implantados ha crecido exponencialmente. A pesar de los avances en las válvulas y su sistema de implantación, las alteraciones de la conducción (AC) siguen siendo la complicación más frecuente. Tienen un impacto negativo en el pronóstico de los pacientes (empeoramiento de la fracción de eyección, necesidad de marcapasos o riesgo aumentado de muerte súbita). Varios factores se han asociado con mayor riesgo de AC, incluyendo bloqueo de rama derecha previo, el tipo de válvula utilizado y el nivel de implantación en el tracto de salida del ventrículo izquierdo.
A pesar de ser la principal complicación tras el TAVI, no existe un consenso general en la estrategia óptima para minimizar la incidencia de AC. En esta revisión se repasará la evidencia disponible en cuanto a la fisiopatología, incidencia, implicaciones clínicas y manejo de las alteraciones de la conducción tras el TAVI.
Aortic stenosis is the most common primary valve disease.1 In high-income countries, the incident cases of rheumatic disease have fallen substantially, and residual valvular diseases are now mostly degenerative, therefore increasing its incidence with age in a population with a longer life expectancy. It affects about 5% of people over 65 years of age.2
Up until recently, the standard treatment for severe symptomatic aortic stenosis was surgical aortic valve replacement with a mechanical or biological prosthesis. However, over the past decade, the use of transcatheter aortic valve implantation (TAVI) has become the option of choice for severe symptomatic aortic stenosis in patients at prohibitive, high3–6 or intermediate surgical risk as estimated by surgical risk scores (Society of Thoracic Surgery score and EuroSCORE II).7–9
The TAVI procedure has increased in numbers over time,10 with its consequent improvement in patient survival through an improvement of the deployment technique that have dramatically decreased associated complications. It has become a routine practice in most centers, with favorable outcomes for the vast majority of patients undergoing the procedure. These results preclude a fast expansion of the number of patients that will benefit from TAVI in the future, including those with low surgical risk and those with different aortic stenosis etiologies.
However, even with the technical development and the minimization of overall complications, conduction disturbances (CD), stroke, bioprosthesis thrombosis and durability are the main setbacks of TAVI.
Conduction disturbances after aortic valve replacement (surgical or transcatheter) is a common occurrence. Studies suggested that new-onset left bundle-branch block (LBBB) has been described in 16-32% and early PPM in approximately 3-8% of patients undergoing surgical aortic valve replacement.11,12 In TAVI population, CD remain one of the most common complications that have not decreased over time even with the development of “second-generation” valves.13 There are pathophysiological explanations for such complications, but the best approach for its avoidance has yet to be determined.
The most common CD after TAVI are new-onset left bundle-branch block (LBBB) and high-degree atrioventricular block (HAVB). These may be transitory or permanent, therefore implying the need for permanent pacemaker (PPM) implantation.14 Knowing the deleterious effect that paced rhythm has on the left ventricular ejection fraction (LVEF), and also the increased risk of sudden death in patients with CD, it is of outmost importance that these complications are limited in number.
In this review we will approach the current evidence for the pathophysiology, incidence, clinical implications and management of CD after TAVI.
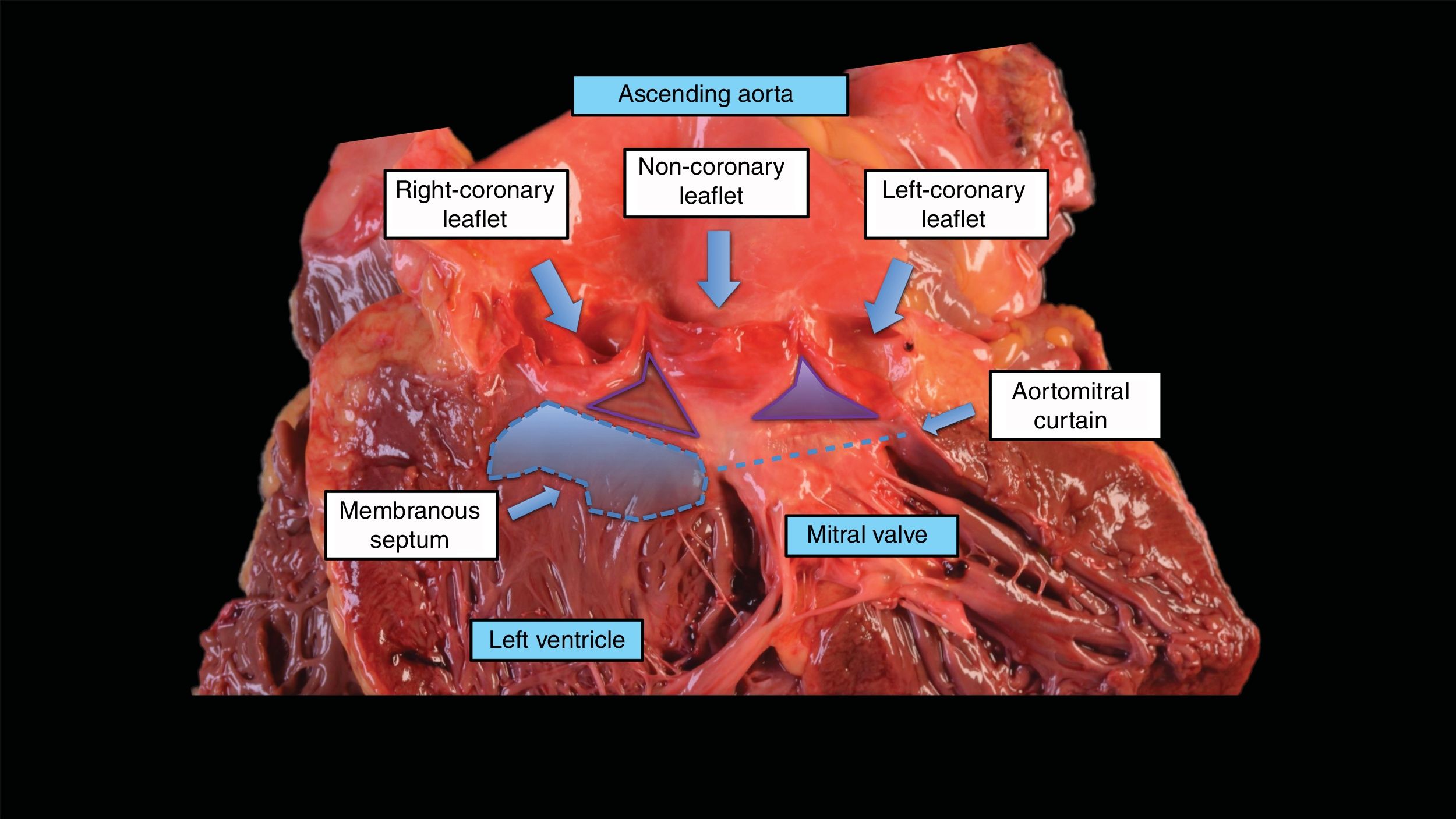
Anatomy of the conduction system and its relationship with transcatheter aortic valve implantationThe origin of conduction alterations is explained by the close relationship between the aortic valve, the left ventricular outflow tract and the conduction system. In the right atrium, the atrioventricular node is located within the triangle of Koch. This triangle is demarcated by the tendon of Todaro, the attachment of the septal leaflet of the tricuspid valve, and the orifice of the coronary sinus. The atrioventricular node is located just inferior to the apex of the triangle adjacent to the membranous septum, and therefore the atrioventricular node is in fact in close proximity to the subaortic region and membranous septum of the left ventricular outflow tract. The atrioventricular node continues as the bundle of His, which penetrates the membranous septum to the left through the central fibrous body. On the left side, the conduction system exits beneath the membranous septum and runs superficially along the crest of the ventricular septum, becoming the fascicles of the left bundle branch (fig. 1). This close relationship between the conduction system, the left ventricular outflow tract and the aortic valve leaflets explains the potential CD after the implantation of an aortic valve.15
The anatomy of the aortic valve, aortic root, coronary leaflets, and the conduction system. Basal attachments of the right and noncoronary leaflets of the aortic valve (arrows), and the location of the membranous septum. The atrioventricular node is in close proximity to the subaortic region and membranous septum of the left ventricular outflow tract.
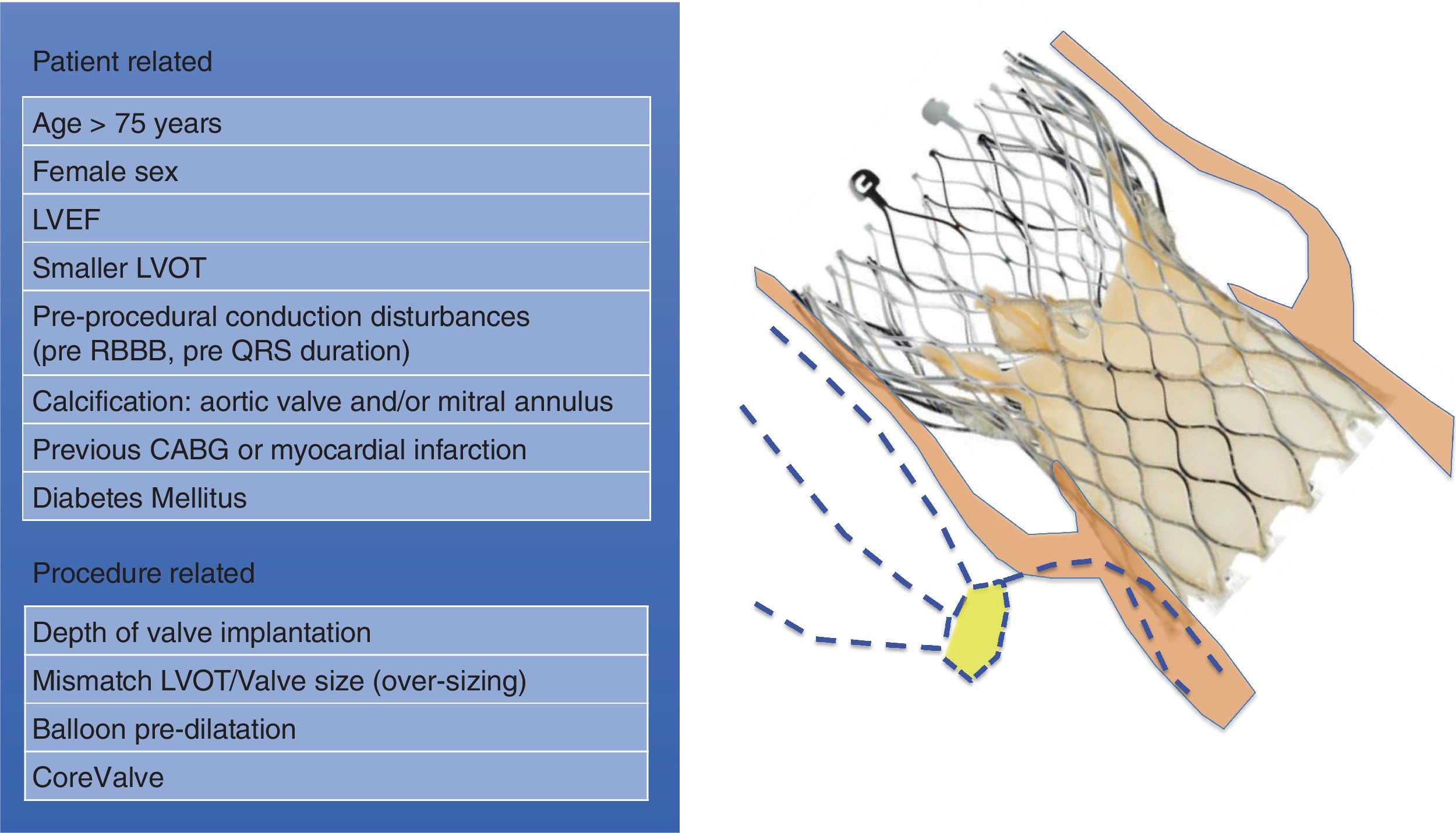
The alteration of the conduction system with a TAVI is explained mainly because of the mechanical insult that the implantation of the valve implies, being able to cause edema, hematoma and ischemia in the interventricular septum. This can also happen during a valvuloplasty, but is more common during the expansion of the bioprosthesis.14 Nevertheless, there are a number of reasons that could be involved in the development of the damage. Regarding the procedure, the depth of the TAVI implantation is the most frequently associated factor, with deeper implantation in the left ventricle outflow tract having a higher risk of CD. The type of prosthesis was found to be a predictor of conduction complications, with the CoreValve prosthesis having a higher risk of new-onset LBBB.14 However, with newer-generation valve systems such as Evolut R and SAPIEN 3, no significant differences were detected between self-expanding and balloon-expandable systems regarding the rate of CD.16 Also, the mismatch between the size of the left ventricular outflow tract and the size of the chosen valve, with the consequent overstretching of the left ventricular outflow tract, has been found to be a strong risk factor for LBBB.16,17 Balloon predilation is often also responsible for triggering CD. Considering the patient-related risk factors for CD after TAVI, various characteristics have been associated with a higher risk: the size of the left ventricular outflow tract, female sex, the presence of pre-procedural CD such as prolonged QRS duration, previous coronary artery bypass graft, diabetes mellitus and the calcification in the aortic valve18–21 (fig. 2).
These conditions highlight the importance of accurate procedure planning and anatomical assessment, in order to identify the potential complications of the procedure and select the most appropriate valve and its size.
Incidence of conduction disturbances after TAVIThere is discordance about the incidence of new-onset LBBB among studies. This may be explained by the different definition of “new-onset” LBBB in these studies, regarding the time of incidence of the CD. Also, different types of valves have been used in each study. Overall, the reported rate of new-onset LBBB is about 27% (4%-65%) of patients with TAVI.22 The incidence of new-onset LBBB has been higher with the use of the first-generation CoreValve. There is still scarce published data regarding the occurrence of new-onset LBBB with new-generation valves, although the results so far are better than with first-generation devices.
After implanting a TAVI, most CD occur in the acute setting, meaning periprocedural or during the first 24h. LBBB can present even in the predilation of the valve, but it normally appears during valve expansion. Subacute LBBB, which is considered from more than 24h after TAVI to discharge, has been reported in a small proportion of patients (2%-8%). Late appearance of LBBB after TAVI (from discharge to 1 year later) is rare and has been described in 0%-3% of patients.23
Pacemaker implantation occurs in 5%-14% of patients with new-onset LBBB during follow-up. The most common indication for PPM is the progression towards HAVB.24 A recent multicenter study including 103 patients with new-onset LBBB post-TAVI was designed to follow these patients and assess their arrhythmic burden.25 An implantable cardiac monitor was implanted after TAVI. Remarkable findings were found: (a) 62% of LBBB persisted at 1-year follow-up; (b) about 50% of the episodes of bradyarrhythmia occurred within the first weeks following TAVR; (c) PPM implantation rate at 1-year follow-up was 10%; (d) HAVB episodes, frequently asymptomatic, were the reason for PPM in 90% of cases; (e) no significant differences were detected between self-expanding and balloon-expandable systems regarding the rate of LBBB, PPM and the recovery rate of CD; and (f) The rate of sudden death was 1% in that study, similar to the sudden death rate at follow-up reported in patients with no prior LBBB in previous studies.26
Clinical implications and prognosisWhile TAVI is usually related to an improvement of LVEF, the development of new-onset LBBB or the need for a pacemaker may induce a decrease in LVEF due to the negative impact it has on atrioventricular and ventricular coupling and reverse LV remodeling. This has an even more important clinical implication for patients with reduced LVEF, and it affects their prognosis.27
LBBB induces dyssynchronous ventricular activation, with delayed left ventricle contraction. This leads to an asynchrony between left and right ventricle. With delayed left ventricle contraction, its diastolic time is decreased, causing a lower output and abnormal septal motion, which ends up in a reduced LVEF. It is known that patients with low LVEF have an increased number of rehospitalizations, which affects their quality of life and have a net impact on prognosis. They also have a higher risk of ventricular arrhythmias and heart failure.28
Apart from the implication in LVEF worsening, LBBB has been associated with increased sudden cardiac death risk29 in patients with QRS longer than 160 milliseconds.26 A plausible explanation would be that the constant compression of the conduction system by the valve could induce the progression of LBBB to HAVB. However, the rate of sudden death in patients with perioperative LBBB treated with new-generation valves results similar to those patients without perioperative LBBB.26
Several studies have compared the morbidity and mortality of patients with and without perioperative LBBB, without consistent results.16,24,29–31 Perioperative (up to 30 days after TAVI) and midterm cardiovascular and all-cause mortality were comparable between the two groups. Regarding morbidity, LBBB patients have a higher incidence of PPM implantation. These results vary among studies, with other groups reporting reduced survival in one year follow-up in patients with new-onset LBBB after TAVI.21,32 Regarding the morbidity, some studies have reported poorer functional status in patients with LBBB and a higher incidence of syncope.16
Between 2% and 51% of patients treated with TAVI will need a new pacemaker implanted during follow up.33 Several groups have studied the impact of the need of PPM after TAVI on mortality, failing to find a significant correlation.30,34 However, the requirement for a PPM may have an impact in the length of hospitalization, thus increasing the rate of further complications and finally reducing the quality of life of these patients. Also, the need for a PPM has a negative effect in the cost-effectiveness of TAVI compared to surgical aortic valve replacement. It is the most expensive complication after TAVI, both because of the cost of the device and also because of the extended hospital admission.
Moreover, there is no evidence to date that supports that the use of newer-generation valves leads to a reduction in either LBBB or PPM implantation, whereas other important complications regarding TAVI and its procedure have been largely reduced.
Management of conduction disturbancesThere is not much evidence available in randomized trials about the best approach regarding the management of CD. The available evidence is observational and mostly retrospective. Thus, the recommendations lack a solid evidence base, and should be considered with caution. The evolution of the ECG in the first hours post-TAVI should be closely monitored.35
Patients with severe calcification of the aortic valve can also present with conduction abnormalities at baseline. Therefore, it should be considered to monitor the patient's ECG before the TAVI procedure, to verify the absence of preprocedural need of PPM. Some patients may present episodes of HAVB or bradycardia, which could worsen with TAVI. In any case, it is unlikely that CD may improve after TAVI, but they may remain unchanged. Even if not caused by TAVI, CD in these patients also need to be prematurely addressed.36 If identified early, these alterations could be promptly treated, therefore decreasing the length of hospitalization. If a CD is present at baseline, the type of valve implanted should be carefully considered, prioritizing valves with a lower risk to induce HAVB (Table 1).
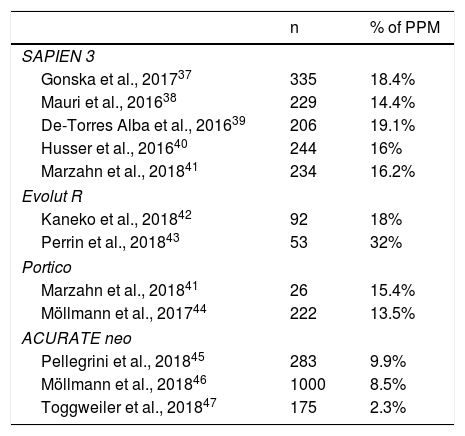
Rates of permanent pacemaker implantation with new-generation TAVI.
| n | % of PPM | |
|---|---|---|
| SAPIEN 3 | ||
| Gonska et al., 201737 | 335 | 18.4% |
| Mauri et al., 201638 | 229 | 14.4% |
| De-Torres Alba et al., 201639 | 206 | 19.1% |
| Husser et al., 201640 | 244 | 16% |
| Marzahn et al., 201841 | 234 | 16.2% |
| Evolut R | ||
| Kaneko et al., 201842 | 92 | 18% |
| Perrin et al., 201843 | 53 | 32% |
| Portico | ||
| Marzahn et al., 201841 | 26 | 15.4% |
| Möllmann et al., 201744 | 222 | 13.5% |
| ACURATE neo | ||
| Pellegrini et al., 201845 | 283 | 9.9% |
| Möllmann et al., 201846 | 1000 | 8.5% |
| Toggweiler et al., 201847 | 175 | 2.3% |
PPM, permanent pacemaker; TAVI, transcatheter aortic valve implantation.
The European Society of Cardiology Guidelines on management of cardiac pacing48 recommend an observational period of 7 days to assess the need for PPM in patients with HAVB after TAVI (class I, level of evidence C). However, in case of complete AV block with low rate of escape rhythm this observation period could be shortened since resolution would be unlikely. Patients should be monitored with telemetry during the length of admission, either in the intensive care unit of the Cardiology ward.
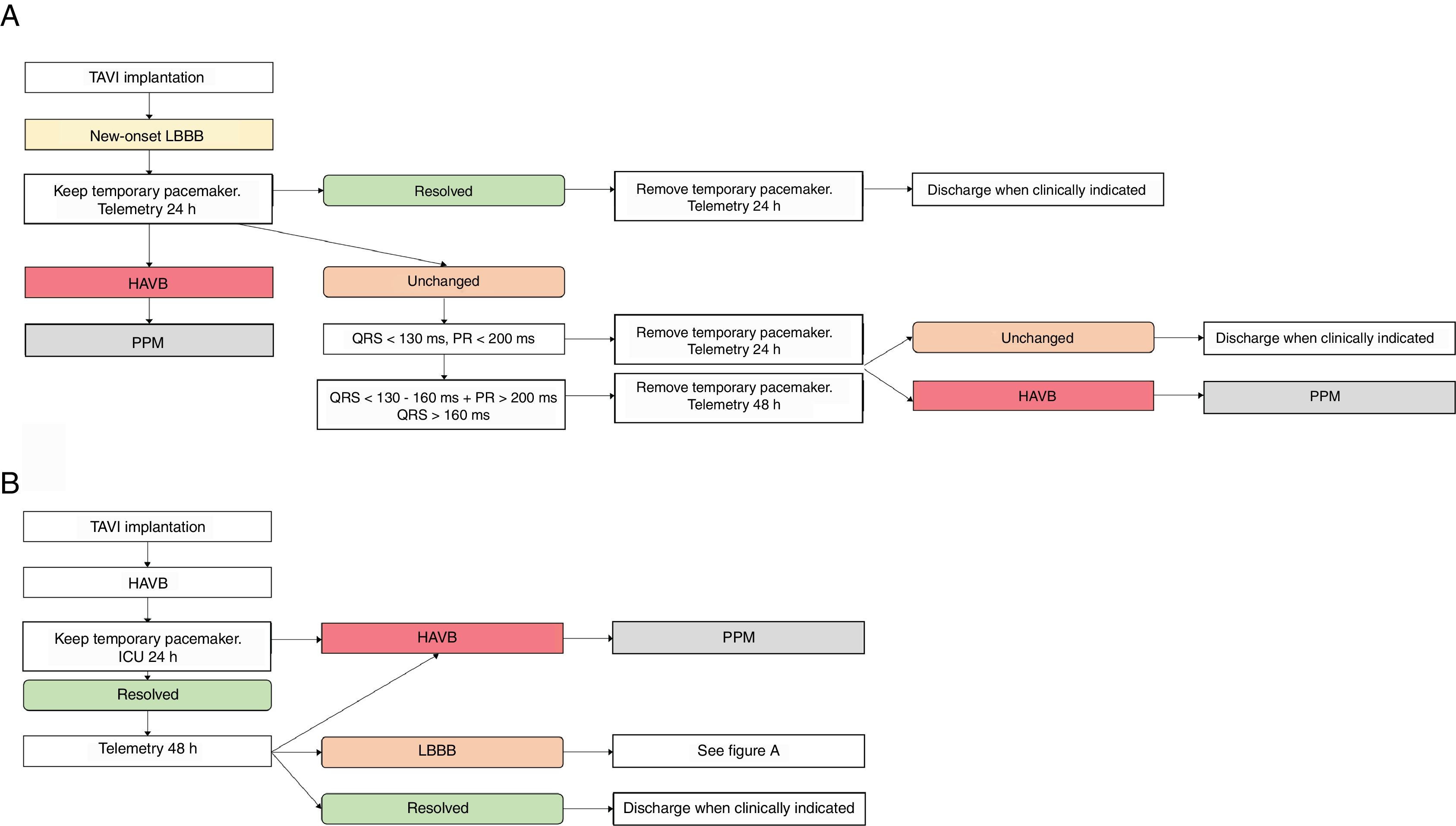
Management of new-onset LBBBThere is lack of evidence in the literature about the best approach in new-onset LBBB. Some algorithms of treatment have been published,35,49 with proposed management of these patients (fig. 3A).
Flowcharts summarizing a suggested approach regarding the management of conduction disturbances: LBBB (A) and High degree AVB (B). HAVB, high-degree atrioventricular block; ICU, intensive care unit; LBBB, left bundle-branch block; TAVI, transcatheter aortic valve implantation procedure; PPM, permanent pacemaker.
The early discharge after TAVI (within 24-48h after the procedure) is not recommended in patients that develop new-onset LBBB. Continuous ECG monitoring should be maintained for 48h to 72h, to detect eventual progression to HAVB. Regarding the temporary pacemaker, it is suggested that it is maintained in an intensive care unit for 24h after the TAVI implantation.49
The progression of LBBB to HAVB is an indication for PPM. However, most patients will persist with the LBBB without progression to more advanced block. There is scarce evidence in the management of these patients. A recent study suggested that a close clinical follow-up during the first month after TAVI and that the implantation of a cardiac monitor in LBBB post-TAVI recipients could be useful leading to a change in treatment in one-third due to an increased burden of arrhythmic events at 1-year follow-up in up to one-half of the patients.25 The use of electrophysiological conduction studies has been explored in some trials, and could be useful to assess CD in patients with LBBB and first degree AV block.50,51 Further evidence is needed, since few patients have been included in the published studies.
Management of HAVBAs stated before, following the European guidelines, a period of clinical observation up to 7 days is indicated in case of CD.
The proportion of patients probably justifies this long length of hospital stay with transient HAVB after TAVI, and by the potential complications and clinical implications of pacemaker implantation (previously stated in this review). However, the consequences of a long hospitalization should also be taken into account. Patients undergoing TAVI are mostly octogenarians with several degrees of frailty and comorbidities. Thus, the immobilization, the risks of a transient pacemaker and the effects on functional recovery should be fully considered.
In order to decrease the length of hospitalization, some groups have explored the option of implanting the PPM the same day of the TAVI, in patients whom develop HAVB. However, a number of patients eventually recover from the CD. Therefore, it would be unnecessary to implant a PPM in these patients, and they would have the added risk of another procedure and of the pacemaker.
In order to address the need of PPM implantation, an intermediate strategy seems adequate to try to minimize the hospital stay and also ascertain that the patient has a permanent CD. Some groups, including ours, have proposed an observation period of 24 h-72h after which a decision is made whether to implant a PPM or not35,49 (fig. 3B).
Regarding the choice of pacemaker, the current guidelines should be followed.48 Whenever possible, spontaneous atrioventricular conduction should be preserved. Resynchronization therapy should be considered in patients when indicated.
New-generation valvesThere is still not enough evidence in the literature regarding the incidence of CD in new-generation valves. These valves have been designed to reduce the incidence of paravalvular leaks found in first-generation valves. However, they seem to fail in decreasing the rate of CD. In fact, the new SAPIEN 3 valve has been found to have a higher PPM rate (14-19%) than its predecessor, the SAPIEN XT (5-12%).3,37–40
Because the development of new valve technology has not particularly addressed the CD induction after TAVI, it seems unlikely that CD will be reduced in the next future (Table 1).
ConclusionsCD are still one of the most common complications after TAVI despite the development of new valves and the evolution of the technique. Conduction alterations may lead to a PPM implantation, which has important clinical and economic consequences and implies an added risk for the patients.
The predictors of CD in TAVI are well known, and include some patient related factors, which cannot be modified, and some procedural factors, which need to be specifically addressed to reduce conduction alterations such as the size of the valve and the type of valve. Thus, the decision should be individualized for each patient.
Further evidence on the pathophysiology of damage into the conduction system induced by TAVI and the effect of new-generation valves and its deployment strategy on the conduction system will improve evidence and patient prognosis.
Conflicts of interestB. Vaquerizo is a consultant for Medtronic Inc. and she has received speaker or consultant honoraria from Abbot Vascular and Boston Scientific. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
We are grateful to Dr. Javier Gimeno Beltrán (Department of Pathology, Hospital del Mar, Barcelona, Spain) and Andrea Sánchez Carpintero (nurse from the Interventional Cardiology Unit, Hospital del Mar, Barcelona, Spain) for their participation in this revision.