Atrial fibrillation (AF) is the most common arrhythmia in the general population and affects 1 out of 5 patients with chronic kidney disease (CKD) not undergoing renal replacement therapy (RRT).1 Renal dysfunction affects haemostasis, leading to both prothrombotic and prohemorrhagic states. The benefits of anticoagulation in the overall population with AF are well known. However, patients with advanced CKD (stages 4 and 5)2 have been systematically excluded from clinical trials studying the efficacy and safety of vitamin K antagonists (VKA) or direct oral anticoagulants (DOACs).3,4 Percutaneous left atrial appendage closure (LAAC) was an alternative for these patients, however there are no specific clinical trials in the CKD setting.2 Therefore, anticoagulation therapy for advanced CKD is challenging.
Our study aimed to describe the clinical characteristics of patients with advanced CKD not undergoing RRT and AF in our environment and whether they received anticoagulant treatment, LAAC, or none. We also evaluated the incidence of thromboembolic and haemorrhagic events.
We designed a retrospective, unicentric, observational study of patients with advanced CKD and previous diagnosis of AF. Patients were included from the Nephrology Outpatient Clinic on 1st January 2020, and were followed-up until 31 December 2021, or they died. The median follow-up time was 24 months (interquartile range [IQR] 17–24). The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital. Patient consent was waived due to the retrospective design during SARS-CoV-2 pandemic situation to avoid putting patients at unnecessary risk. Information was obtained from clinical records. The incidence of thromboembolic events, major bleeding (requiring a visit to the emergency department and/or hospitalization) and mortality was recorded.
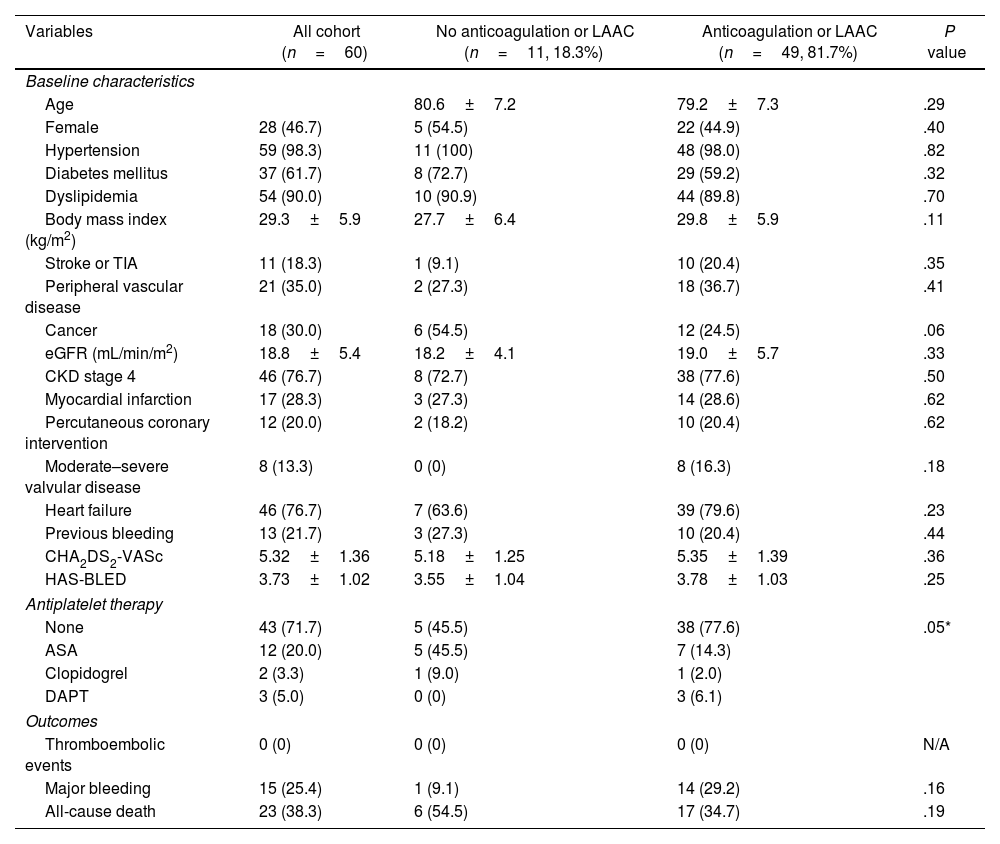
A total of 60 patients were included; 53.3% of them were male, and the mean age was 80±7 years. AF was permanent/persistent in 61.7% of the patients. The estimated glomerular filtration rate (eGFR) was 18.8±5.4mL/min/m2. Moderate–severe valvular disease was present in 13.3%, but none of them had mitral stenosis, rheumatic valvulopathy or mechanical prostheses. The 18.3% of the patients had a history of stroke, most of whom were ischaemic, and 21.7% had a history of prior bleeding (70% from gastrointestinal sources). The remaining baseline patient characteristics are presented in Table 1.
Baseline characteristics and clinical outcomes between patients treated with anticoagulation or left atrial appendage closure compared to those without.
| Variables | All cohort (n=60) | No anticoagulation or LAAC (n=11, 18.3%) | Anticoagulation or LAAC (n=49, 81.7%) | P value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age | 80.6±7.2 | 79.2±7.3 | .29 | |
| Female | 28 (46.7) | 5 (54.5) | 22 (44.9) | .40 |
| Hypertension | 59 (98.3) | 11 (100) | 48 (98.0) | .82 |
| Diabetes mellitus | 37 (61.7) | 8 (72.7) | 29 (59.2) | .32 |
| Dyslipidemia | 54 (90.0) | 10 (90.9) | 44 (89.8) | .70 |
| Body mass index (kg/m2) | 29.3±5.9 | 27.7±6.4 | 29.8±5.9 | .11 |
| Stroke or TIA | 11 (18.3) | 1 (9.1) | 10 (20.4) | .35 |
| Peripheral vascular disease | 21 (35.0) | 2 (27.3) | 18 (36.7) | .41 |
| Cancer | 18 (30.0) | 6 (54.5) | 12 (24.5) | .06 |
| eGFR (mL/min/m2) | 18.8±5.4 | 18.2±4.1 | 19.0±5.7 | .33 |
| CKD stage 4 | 46 (76.7) | 8 (72.7) | 38 (77.6) | .50 |
| Myocardial infarction | 17 (28.3) | 3 (27.3) | 14 (28.6) | .62 |
| Percutaneous coronary intervention | 12 (20.0) | 2 (18.2) | 10 (20.4) | .62 |
| Moderate–severe valvular disease | 8 (13.3) | 0 (0) | 8 (16.3) | .18 |
| Heart failure | 46 (76.7) | 7 (63.6) | 39 (79.6) | .23 |
| Previous bleeding | 13 (21.7) | 3 (27.3) | 10 (20.4) | .44 |
| CHA2DS2-VASc | 5.32±1.36 | 5.18±1.25 | 5.35±1.39 | .36 |
| HAS-BLED | 3.73±1.02 | 3.55±1.04 | 3.78±1.03 | .25 |
| Antiplatelet therapy | ||||
| None | 43 (71.7) | 5 (45.5) | 38 (77.6) | .05* |
| ASA | 12 (20.0) | 5 (45.5) | 7 (14.3) | |
| Clopidogrel | 2 (3.3) | 1 (9.0) | 1 (2.0) | |
| DAPT | 3 (5.0) | 0 (0) | 3 (6.1) | |
| Outcomes | ||||
| Thromboembolic events | 0 (0) | 0 (0) | 0 (0) | N/A |
| Major bleeding | 15 (25.4) | 1 (9.1) | 14 (29.2) | .16 |
| All-cause death | 23 (38.3) | 6 (54.5) | 17 (34.7) | .19 |
ASA, acetylsalicylic acid; CKD, chronic kidney disease; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; LAAC, left atrial appendage closure; TIA, transient ischaemic attack.
Most patients (81.7%) received anticoagulant treatment or LAAC. The 2 groups had no significant differences in the CHA2DS2-VASc and HAS-BLED scores. Although not statistically significant, stroke and valve disease prevalence were higher in the treatment group, whereas cancer was less prevalent. The reasons reported in the medical records for not initiating anticoagulation were paroxysmal atrial fibrillation in the context of acute severe disease with no recurrence (36%), prior bleeding (19%), frailty (9%), renal dysfunction (9%), and unknown (27%). Antiplatelet treatment was more frequent in the non-treatment group.
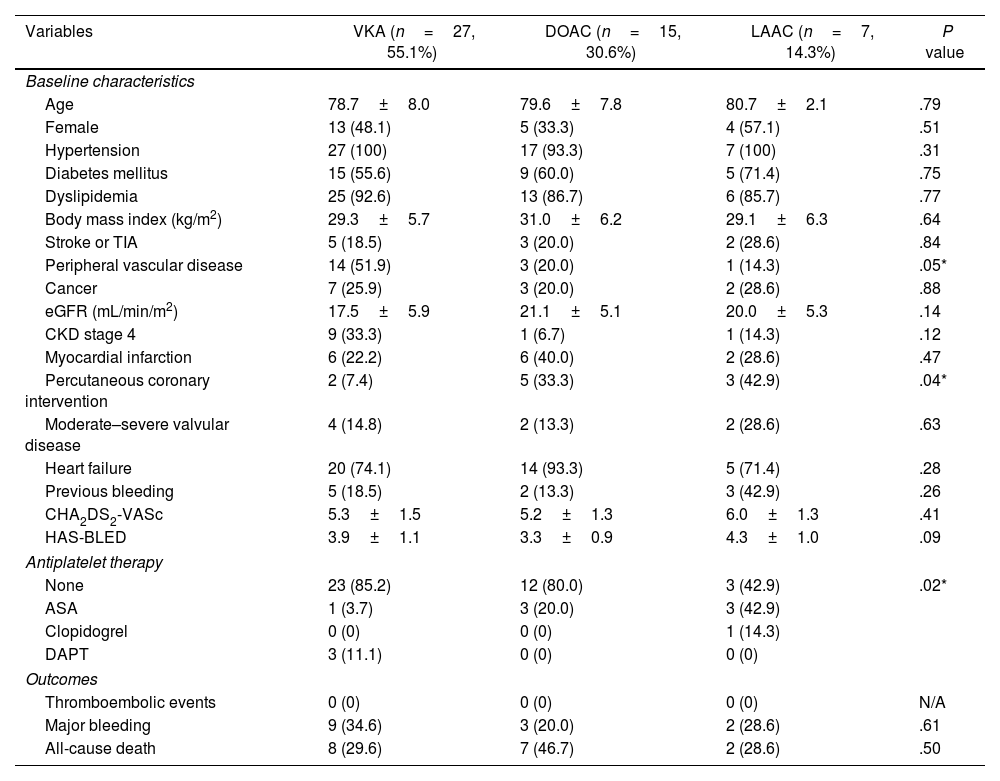
VKA therapy was the most used therapy, followed by DOACs and LAAC. The baseline characteristics of patients according to treatment are presented in Table 2. A lower eGFR was observed in the VKA group, but the difference was not statistically significant. Of the patients undergoing VKA treatment, 55.6% had a labile international normalized ratio (INR) (>60% of the time out of the therapeutic range). Within the DOAC group, 80% received apixaban and the rest received rivaroxaban, with a properly adjusted dose in 87% of cases.
Baseline characteristics and clinical outcomes between patients with anticoagulation or left atrial appendage closure depending on the treatment.
| Variables | VKA (n=27, 55.1%) | DOAC (n=15, 30.6%) | LAAC (n=7, 14.3%) | P value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age | 78.7±8.0 | 79.6±7.8 | 80.7±2.1 | .79 |
| Female | 13 (48.1) | 5 (33.3) | 4 (57.1) | .51 |
| Hypertension | 27 (100) | 17 (93.3) | 7 (100) | .31 |
| Diabetes mellitus | 15 (55.6) | 9 (60.0) | 5 (71.4) | .75 |
| Dyslipidemia | 25 (92.6) | 13 (86.7) | 6 (85.7) | .77 |
| Body mass index (kg/m2) | 29.3±5.7 | 31.0±6.2 | 29.1±6.3 | .64 |
| Stroke or TIA | 5 (18.5) | 3 (20.0) | 2 (28.6) | .84 |
| Peripheral vascular disease | 14 (51.9) | 3 (20.0) | 1 (14.3) | .05* |
| Cancer | 7 (25.9) | 3 (20.0) | 2 (28.6) | .88 |
| eGFR (mL/min/m2) | 17.5±5.9 | 21.1±5.1 | 20.0±5.3 | .14 |
| CKD stage 4 | 9 (33.3) | 1 (6.7) | 1 (14.3) | .12 |
| Myocardial infarction | 6 (22.2) | 6 (40.0) | 2 (28.6) | .47 |
| Percutaneous coronary intervention | 2 (7.4) | 5 (33.3) | 3 (42.9) | .04* |
| Moderate–severe valvular disease | 4 (14.8) | 2 (13.3) | 2 (28.6) | .63 |
| Heart failure | 20 (74.1) | 14 (93.3) | 5 (71.4) | .28 |
| Previous bleeding | 5 (18.5) | 2 (13.3) | 3 (42.9) | .26 |
| CHA2DS2-VASc | 5.3±1.5 | 5.2±1.3 | 6.0±1.3 | .41 |
| HAS-BLED | 3.9±1.1 | 3.3±0.9 | 4.3±1.0 | .09 |
| Antiplatelet therapy | ||||
| None | 23 (85.2) | 12 (80.0) | 3 (42.9) | .02* |
| ASA | 1 (3.7) | 3 (20.0) | 3 (42.9) | |
| Clopidogrel | 0 (0) | 0 (0) | 1 (14.3) | |
| DAPT | 3 (11.1) | 0 (0) | 0 (0) | |
| Outcomes | ||||
| Thromboembolic events | 0 (0) | 0 (0) | 0 (0) | N/A |
| Major bleeding | 9 (34.6) | 3 (20.0) | 2 (28.6) | .61 |
| All-cause death | 8 (29.6) | 7 (46.7) | 2 (28.6) | .50 |
ASA, acetylsalicylic acid; CKD, chronic kidney disease; DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; LAAC, left atrial appendage closure; TIA, transient ischaemic attack; VKA, vitamin-K-antagonists.
Patients who underwent LAAC had a significantly higher prevalence of previous percutaneous coronary intervention and antiplatelet therapy, which included acetylsalicylic acid for 1–9 months or indefinite treatment in cases of ischaemic heart disease. They had a higher prevalence of prior bleeding, although not statistically significant. CHA2DS2-VASc and HAS-BLED scores were higher, but differences were not statistically significant.
No thromboembolic events were detected in either group during the 2 year follow-up period. There was a trend towards a higher incidence of bleeding in the anticoagulant treatment group compared to the non-treatment group, particularly in the VKA group, but without reaching statistical significance. Gastrointestinal bleeding was the most frequent complication. The overall mortality rate was 38.3%, which was higher but not statistically significant in the non-treatment group. Out of these deaths, 2 were due to haemorrhagic causes (intracranial bleeding), both of which occurred during anticoagulant treatment (VKA and DOAC, respectively).
CKD and AF share a bidirectional relationship, as the presence of one increases the likelihood of developing the other and increases the risk of thromboembolism, bleeding, and mortality. Anticoagulation in patients with advanced CKD is challenging in clinical practice.
In our study, over 80% of the patients received anticoagulant treatment or LAAC. A recent Korean registry of 260 patients with stage 4 and 5 CKD or undergoing haemodialysis reported an anticoagulation rate of 60%.5 We believe that the presence of a cohort with structured hospital follow-up could partially explain the high prevalence of anticoagulation therapy in our study. No significant differences were found between the treated and non-treated groups. However, there was a tendency to treat patients with previous ischaemic events.
VKA is the most common anticoagulation treatment for patients with CKD, including in our study. However, its narrow therapeutic range and multiple interactions complicate its management. Patients with advanced CKD have more frequent out-of-range INR control, leading to a higher risk of thromboembolism, bleeding, and mortality.2,3 In our cohort, more than half of the patients showed poor INR control. Complications of VKAs, such as vascular calcification progression, arterial stiffness, calciphylaxis, and anticoagulant-related nephropathy, are more common in the CKD population.1
The efficacy and safety of DOACs compared to VKAs have been demonstrated in pivotal clinical trials. Still, these trials excluded patients with an eGFR<30mL/min/m2 (except for apixaban, which excluded patients with an eGFR<25mL/min/m2). Therefore, results obtained in patients without CKD or with moderate stages (where benefits have been demonstrated), and results from observational studies with contradictory findings need to be extrapolated to advanced CKD setting.3,4 The renal excretion rate of these drugs ranges from 80% for dabigatran to 27% for apixaban, requiring dose adjustment. Regulatory agencies and clinical practice guidelines have provided recommendations regarding this issue, but there are some differences in advanced CKD.2,3 In our study, nearly one-third of the patients received DOACs. Rivaroxaban and apixaban were the chosen DOACs since they are the drugs, along with edoxaban, with approved use in patients with the lowest eGFR.1 Regular monitoring of renal function was essential because a decline in eGFR may contraindicate these drugs.
LAAC represents an alternative for patients at high bleeding risk, but there are no specific clinical trials in CKD. Observational studies have associated advanced CKD with a higher rate of in-hospital complications than patients without CKD.6 However, the antithrombotic regimen after the procedure may increase the risk of bleeding in patients with advanced CKD.3
During follow-up, no thromboembolic events were detected, although nearly 20% of patients had a history of stroke. One-fourth of the overall cohort experienced major bleeding, highlighting the risk of bleeding in this population. Patients receiving anticoagulant treatment or LAAC bled more than those who did not receive therapy, particularly in the VKA group. The incidence of bleeding in the LAAC group was also high, probably because of antiplatelet therapy. Additionally, the overall mortality rate was high, which can be attributed to advanced age and high prevalence of comorbidities.
The main limitations of our study are its sample size due to its unicentric design and its retrospective design, which limit the statistical power and the applicability of our results.
Anticoagulant treatment in patients with AF and advanced CKD is an area with multiple uncertainties owing to the lack of strong support for a specific strategy. With increasing life expectancy and cardiovascular risk factors, the population with AF and CKD is expected to increase. There is a need for clinical trials exploring the benefits of anticoagulation and evaluating the efficacy and safety of different anticoagulant regimens in these patients.
FundingThis research received no external funding from public agencies, commercial sectors, or non-profit organizations.
Ethical considerationsThe study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital. Patient consent was waived due to the retrospective design during SARS-CoV-2 pandemic situation to avoid putting patients at unnecessary risk. The article adheres to the STROBE statement. The authors followed SAGER guidelines for addressing sex and gender variables.
Statement on the use of artificial intelligenceNo artificial intelligence has been used at any stage in the development of this scientific project.
Authors’ contributionsS. Valdivielso Moré and I. Galcerán Herrera oversaw conceptualization and methodology. Software was overseen by A. García Alonso, M. Vicente Elcano and I. Galcerán Herrera. Validation was assigned to S. Valdivielso Moré and N. Farré. N. Farré oversaw formal analysis. Investigation and Data Curation were assigned to A. García Alonso, M. Vicente Elcano and I. Galcerán Herrera. A. García Alonso oversaw writing and original draft preparation. A. García Alonso, N. Farré, S. Ruiz Bustillo, and S. Valdivielso Moré participated in writing, review and editing of the work. S. Valdivielso Moré and N. Farré supervised the work. S. Valdivielso Moré and N. Farré oversaw project administration. All authors have read and agreed to the published version of the manuscript.
Conflicts of interestThe authors declare no conflict of interest.