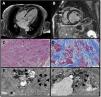
A 64-year-old female was diagnosed in 1987 with cutaneous lupus erythematosus and treated with chloroquine (alternating with hydroxychloroquine) and corticoids for 10 years. In 2019, she was admitted for heart failure, moderate left ventricular dysfunction (left ventricular ejection fraction 40%), and severe mitral regurgitation without significant coronary artery disease. Uneventful mitral valve replacement was performed, but 3 months after discharge she was re-hospitalized with overt congestive heart failure. Mitral prosthesis function was normal but cardiac magnetic resonance showed a severely reduced left ventricular ejection fraction of 24% and extensive patchy sub-epicardial fibrosis (Fig. 1A and B). Endomyocardial biopsy was performed, confirming massive diffuse fibrosis (Fig. 1C and D: haematoxylin–eosin stain and Masson's Trichrome stain). Electron microscopy revealed myeloid and curvilinear bodies (Fig. 1E and F: thick arrowheads), which are highly suggestive of cardiac toxicity by antimalarial drugs. Fabry disease was ruled out and Congo Red Staining was negative. The patient died within months of diagnosis.
In addition to their value for malaria treatment and prophylaxis, chloroquine and hydroxychloroquine mildly suppress the immune system and are used in some autoimmune disorders, such as rheumatoid arthritis and lupus erythematosus. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of chloroquine-mediated cardiotoxicity. Chloroquine may be an important unsuspected contributing cause of cardiac dysfunction in patients with rheumatological disease. Cardiac magnetic resonance imaging and, most importantly, endomyocardial biopsy should be considered early for diagnosis and management. Increased awareness of cardiac dysfunction in patients undergoing long-term chloroquine treatment is advisable. At diagnosis time, written informed consent was obtained from patient for publication of this case report.
FundingNone
Conflicts of interest:None