Temporary pacemaker (TP) is used in patients with high-degree atrioventricular block (HAVB) and ST-segment elevation myocardial infarction (STEMI) to maintain cardiac output and prevent the onset of malignant arrhythmias. Our aim was to characterize the outcomes of patients with STEMI who require TP implantation in the primary angioplasty era.
MethodsWe enrolled all consecutive patients with STEMI who underwent primary angioplasty from 2004 to 2017 in a tertiary care referral center. Patients with STEMI and HAVB who required TP implantation were analyzed. Patients with anterior and inferior STEMI were compared and a multivariate analysis was performed to identify mortality risk factors. All patients completed 30-day and 1-year follow-up.
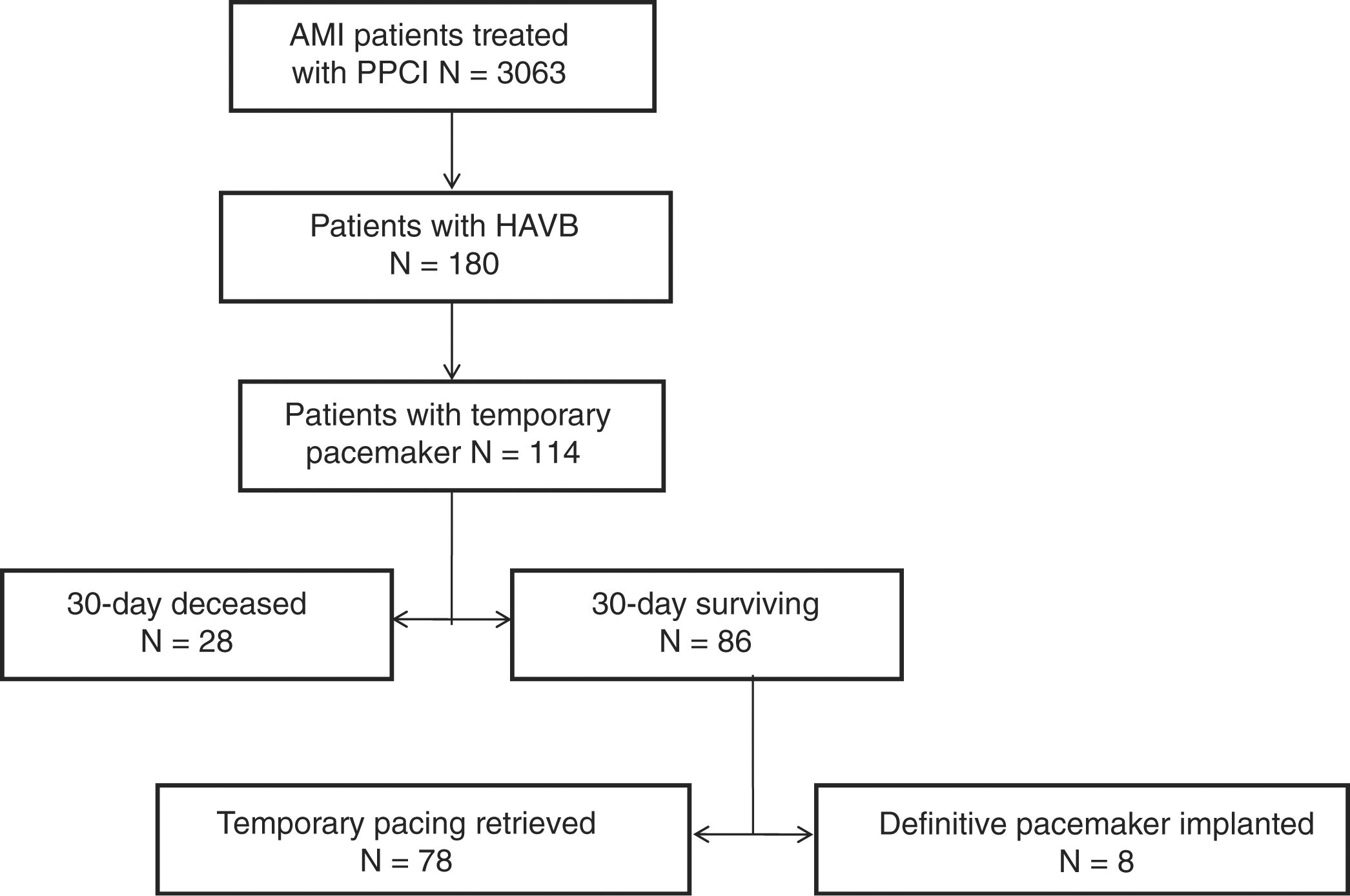
ResultsOf the 3063 patients in the study cohort, 180 (5.9%) had HAVB at the time of cardiac catheterization, and 114 (3.7%) underwent TP implantation. Thirty-day and 1-year mortality were 25.6% and 31.5%, respectively. Low left ventricle ejection fraction and impaired renal function showed independent association with mortality in this patient population. Patients with anterior STEMI who required TP had a ∼4-fold higher 30-day mortality than those with inferior STEMI (71% vs 18%; P≤.001). Only in 7% of patients a definitive pacemaker was finally implanted.
ConclusionsPatients with STEMI and HAVB who require TP implantation, particularly those with anterior STEMI, are at high risk of adverse outcome. Low left ventricle ejection fraction and impaired renal function are independently associated with mortality. A definitive pacemaker implant is finally required in a small proportion of this patient population.
El marcapasos temporal (MT) se emplea en los pacientes con infarto agudo de miocardio con elevación del segmento ST (IAMCEST) y bloqueo auriculoventricular de alto grado (BAVAG) para mantener el gasto cardiaco y evitar la aparición de arritmias ventriculares malignas. Nuestro objetivo fue evaluar el pronóstico de los pacientes con IAMCEST que requieren MT en la era de la angioplastia primaria.
MétodosSe incluyeron de forma consecutiva todos los pacientes con IAMCEST a los que se realizó angioplastia primaria desde 2004 a 2017 en un centro terciario de referencia. Se analizaron los pacientes con IAMCEST y BAVAG que requirieron MT. Se compararon los pacientes con IAMCEST anterior e inferior y se realizó un análisis multivariado para identificar factores de riesgo de mortalidad. Todos los pacientes completaron un seguimiento a 30 días y un año.
ResultadosDe los 3.063 pacientes de la cohorte, 180 (5,9%) tenían BAVAG en el momento de la coronariografía, y 114 (3,7%) requirieron MT. La mortalidad a 30 días y un año fue del 25,6 y 31,5%, respectivamente. La disfunción ventricular izquierda y la insuficiencia renal se asociaron de forma independiente con la mortalidad. Los pacientes con IAMCEST anterior que requirieron MT tuvieron una mortalidad a 30 días, casi cuatro veces superior a los pacientes con IAMCEST inferior (71 frente a 18%; p ≤ 0,001). Solo un 7% de los pacientes requirieron implante de un marcapasos definitivo.
ConclusionesLos pacientes con IAMCEST y BAVAG que requieren MT, en particular aquellos con infarto anterior, muestran un riesgo elevado de complicaciones y un peor pronóstico. La fracción de eyección disminuida y la insuficiencia renal son factores independientes de mayor mortalidad. Solo una pequeña proporción de estos pacientes necesitará el implante de un marcapasos definitivo.
High degree atrioventricular block (HAVB) is a frequent complication in ST-segment elevation myocardial infarction (STEMI), which occasionally requires a temporary pacemaker (TP) insertion to maintain cardiac output and to prevent the onset of malignant arrhythmias in the acute phase.1–3 Previous studies have focused on the evolution and prognosis of HAVB following STEMI,1 whereas the role of TP has not been addressed since the early small series,4,5 The purpose of the present study is to characterize the incidence and outcome of patients with STEMI and HAVB who require TP insertion in the current primary percutaneous coronary intervention (PPCI) era. We also aimed to identify potential prognostic factors and differences according to the STEMI location.
MethodsWe enrolled all consecutive patients with STEMI admitted to a tertiary care referral center for PPCI from 2004 to 2017. The study was approved by the local Ethics Committee of Clinical Research (Hospital Clínico San Carlos). All patients provided informed consent. Our center had an acute cardiac care unit managed by cardiologists since the beginning of the study period. Only patients with STEMI and HAVB who required therapeutic TP insertion were analyzed. During hospitalization, all demographic, clinical, electrocardiographic, echocardiographic and angiographic variables were collected. All patients alive at discharge completed a 1-year follow-up.
STEMI was defined according to the guidelines.6 HAVB was defined as second or third-degree atrioventricular block. The presence of atrioventricular conduction disorders was documented through a standard, 12-lead electrocardiogram in all cases. Electrocardiogram was taken at the time of STEMI-diagnosis, every eight hours after PPCI during the first 24h and daily thereafter.
TP was implanted in case of hemodynamic instability and HAVB with heart rate of ≤ 40bpm refractory to intravenous atropine, isoproterenol and dopamine administration according to our local protocol. The decision and time to start pacing stimulation was left to the treating medical team in each case. In all cases, a fluoroscopy-guided transfemoral approach was used to place the pacemaker electrode into the right ventricle apex. Conduction recovery was assessed daily after revascularization, up to one week before permanent pacemaker implantation.
Unsuccessful PPCI was considered when the PPCI procedure resulted in Thrombolysis in Myocardial Infarction flow grade 0 or I, irrespective of the residual stenosis. Total ischemic time was defined as the time between symptom onset and balloon inflation. Renal insufficiency was defined as a creatinine clearance of less than 60mL/min/1.73m2.
We assessed 30-day and 1-year mortality and in-hospital complications.
Statistical analysisQuantitative variables are expressed as mean±standard deviation, and differences were analyzed with the Student's t test. Categorical variables are reported as a frequency and percentage and compared with the chi-square test and Fisher's exact test when appropriate. Differences were considered statistically significant at P value<.05. A multivariate logistic regression analysis was performed to identify risk factors of 30-day mortality in the whole cohort and in those patients with inferior STEMI. We included in the model those variables previously known to be associated with mortality and those which resulted statistically significant in the univariate analysis. The adjusted odds ratios (OR) with 95% confidence intervals (95%CI) have been calculated. Survival analysis was performed with the Kaplan Meyer method and differences were assessed with the log-rank test. All tests were 2-tailed. IBM SPSS Statistics V22.0 software was used for the statistical analysis.
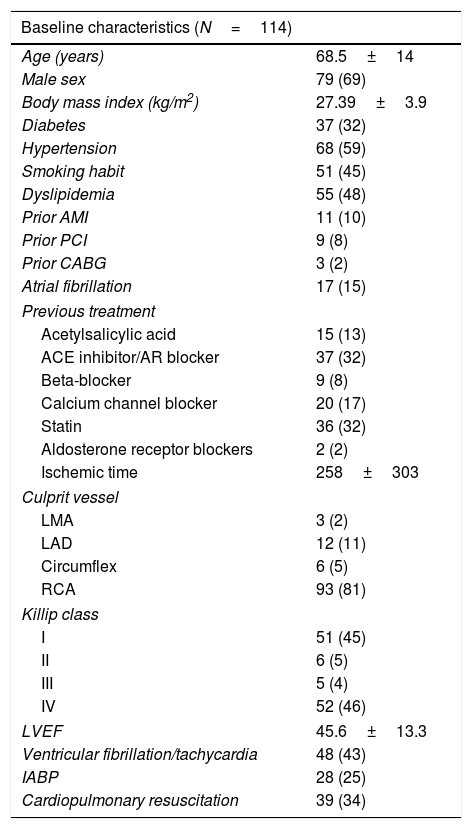
ResultsOf 3063 patients in the study cohort, 180 (5.9%) had HAVB at the time of cardiac catheterization, and 114 (3.7%) required TP implantation (Fig. 1). Baseline characteristics of the study population are shown in Table 1. There was a high prevalence of cardiovascular risk factors among patients and inferior location of STEMI was the most frequently observed (87%) (Table 1).
Baseline clinical, echocardiographic and angiographic characteristics.
| Baseline characteristics (N=114) | |
|---|---|
| Age (years) | 68.5±14 |
| Male sex | 79 (69) |
| Body mass index (kg/m2) | 27.39±3.9 |
| Diabetes | 37 (32) |
| Hypertension | 68 (59) |
| Smoking habit | 51 (45) |
| Dyslipidemia | 55 (48) |
| Prior AMI | 11 (10) |
| Prior PCI | 9 (8) |
| Prior CABG | 3 (2) |
| Atrial fibrillation | 17 (15) |
| Previous treatment | |
| Acetylsalicylic acid | 15 (13) |
| ACE inhibitor/AR blocker | 37 (32) |
| Beta-blocker | 9 (8) |
| Calcium channel blocker | 20 (17) |
| Statin | 36 (32) |
| Aldosterone receptor blockers | 2 (2) |
| Ischemic time | 258±303 |
| Culprit vessel | |
| LMA | 3 (2) |
| LAD | 12 (11) |
| Circumflex | 6 (5) |
| RCA | 93 (81) |
| Killip class | |
| I | 51 (45) |
| II | 6 (5) |
| III | 5 (4) |
| IV | 52 (46) |
| LVEF | 45.6±13.3 |
| Ventricular fibrillation/tachycardia | 48 (43) |
| IABP | 28 (25) |
| Cardiopulmonary resuscitation | 39 (34) |
ACE inhibitor, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; AR, angiotensin receptor; CABG, coronary artery bypass graft surgery; IABP, intra-aortic balloon pump; LAD, left anterior descending artery; LMA, left main artery; LVEF, left ventricle ejection fraction; PCI, percutaneous coronary intervention; RCA, right coronary artery.
Data are expressed as no. (%) or mean±standard deviation.
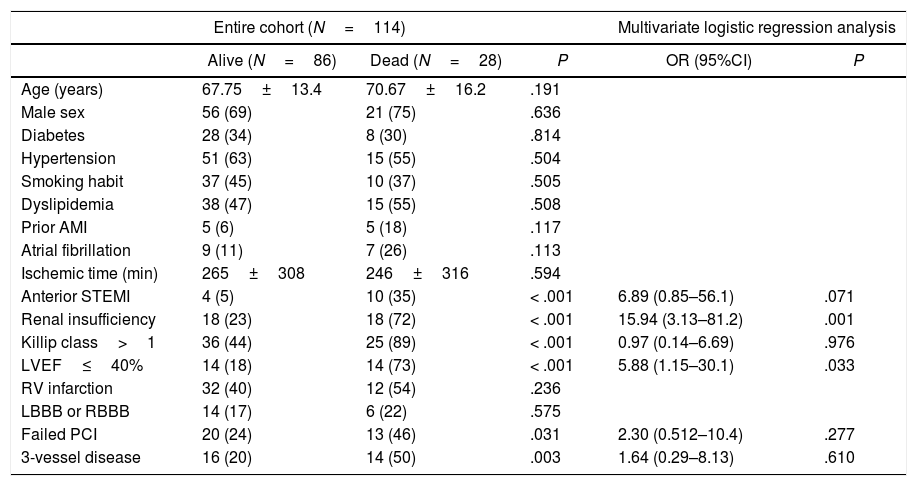
Thirty-day and 1-year mortality were 25.6% and 31.5%, respectively. Main causes of death were refractory shock in most patients (82%), ventricular arrhythmias in 15%, and non-cardiac causes in 3% of cases. Anterior location of STEMI, Killip class, left ventricular ejection fraction (LVEF), renal failure, failed PPCI, and 3-vessel disease were associated with a higher 30-day mortality in the univariate analysis (Table 2). However, only low LVEF and renal failure were independently associated with 30-day mortality in the multivariate logistic regression analysis (Table 2).
Thirty-day mortality in the entire cohort and inferior STEMI patients.
| Entire cohort (N=114) | Multivariate logistic regression analysis | ||||
|---|---|---|---|---|---|
| Alive (N=86) | Dead (N=28) | P | OR (95%CI) | P | |
| Age (years) | 67.75±13.4 | 70.67±16.2 | .191 | ||
| Male sex | 56 (69) | 21 (75) | .636 | ||
| Diabetes | 28 (34) | 8 (30) | .814 | ||
| Hypertension | 51 (63) | 15 (55) | .504 | ||
| Smoking habit | 37 (45) | 10 (37) | .505 | ||
| Dyslipidemia | 38 (47) | 15 (55) | .508 | ||
| Prior AMI | 5 (6) | 5 (18) | .117 | ||
| Atrial fibrillation | 9 (11) | 7 (26) | .113 | ||
| Ischemic time (min) | 265±308 | 246±316 | .594 | ||
| Anterior STEMI | 4 (5) | 10 (35) | < .001 | 6.89 (0.85–56.1) | .071 |
| Renal insufficiency | 18 (23) | 18 (72) | < .001 | 15.94 (3.13–81.2) | .001 |
| Killip class>1 | 36 (44) | 25 (89) | < .001 | 0.97 (0.14–6.69) | .976 |
| LVEF≤40% | 14 (18) | 14 (73) | < .001 | 5.88 (1.15–30.1) | .033 |
| RV infarction | 32 (40) | 12 (54) | .236 | ||
| LBBB or RBBB | 14 (17) | 6 (22) | .575 | ||
| Failed PCI | 20 (24) | 13 (46) | .031 | 2.30 (0.512–10.4) | .277 |
| 3-vessel disease | 16 (20) | 14 (50) | .003 | 1.64 (0.29–8.13) | .610 |
| Inferior STEMI (N=100) | Multivariate logistic regression analysis | ||||
|---|---|---|---|---|---|
| Alive (N=82) | Dead (N=18) | P | OR (95%CI) | P | |
| Age (years) | 67.3±13.2 | 74.4±15.7 | .051 | 1.02 (0.94–1.11) | .594 |
| Male sex | 55 (71) | 11 (61) | .392 | ||
| Diabetes | 28 (36) | 5 (29) | .587 | ||
| Hypertension | 48 (62) | 13 (76) | .269 | ||
| Smoking habit | 36 (46) | 3 (17) | .027 | ||
| Dyslipidemia | 37 (48) | 10 (58) | .421 | ||
| Prior AMI | 5 (6.3) | 4 (23) | .053 | ||
| Atrial fibrillation | 8 (10) | 5 (29) | .055 | ||
| Ischemic time (minutes) | 267±314 | 269±386 | .981 | ||
| Renal insufficiency | 17 (23) | 13 (81) | < .001 | 27.3 (3.06–243.4) | .003 |
| Killip class>1 | 32 (41) | 15 (83) | .002 | 1.4 (0.16–12.2) | .763 |
| LVEF≤40 | 10 (13) | 5 (50) | .013 | 16.09 (1.11–33.5) | .038 |
| RV infarction | 31 (41) | 12 (80) | .005 | 1.82 (0.33–10.2) | .494 |
| LBBB or RBBB | 11 (14) | 2 (11) | .999 | ||
| Failed PCI | 19 (24) | 8 (44) | .144 | 2.25 (0.38–13.1) | .370 |
| 3-vessel disease | 15 (19) | 8 (44) | .035 | 2.49 (0.48–12.8) | .276 |
AMI, acute myocardial infarction; LVEF, left ventricle ejection fraction; LBBB, left bundle branch block; PCI, percutaneous coronary intervention; RBBB, right bundle branch block, RV, right ventricle; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as no. (%) or mean±standard deviation.
Focusing on patients with inferior STEMI who required TP insertion, those who died were older, and had a higher incidence of right ventricle infarction, a worse Killip class, lower LVEF, worse renal function, and higher incidence of 3-vessel disease. Nevertheless, only low LVEF (≤ 40%) and renal insufficiency remained independently associated with 30-day mortality in the multivariate analysis (Table 2).
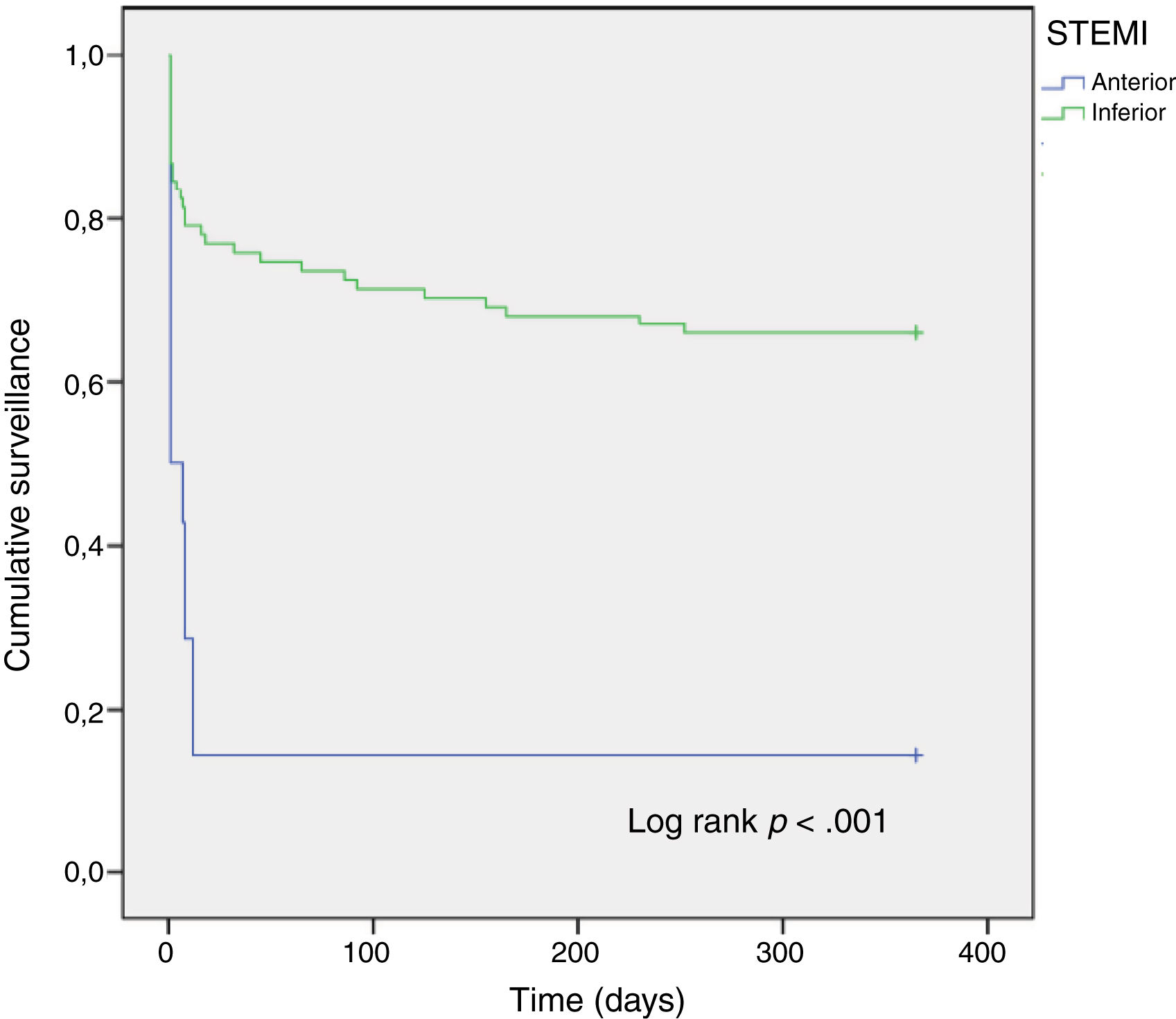
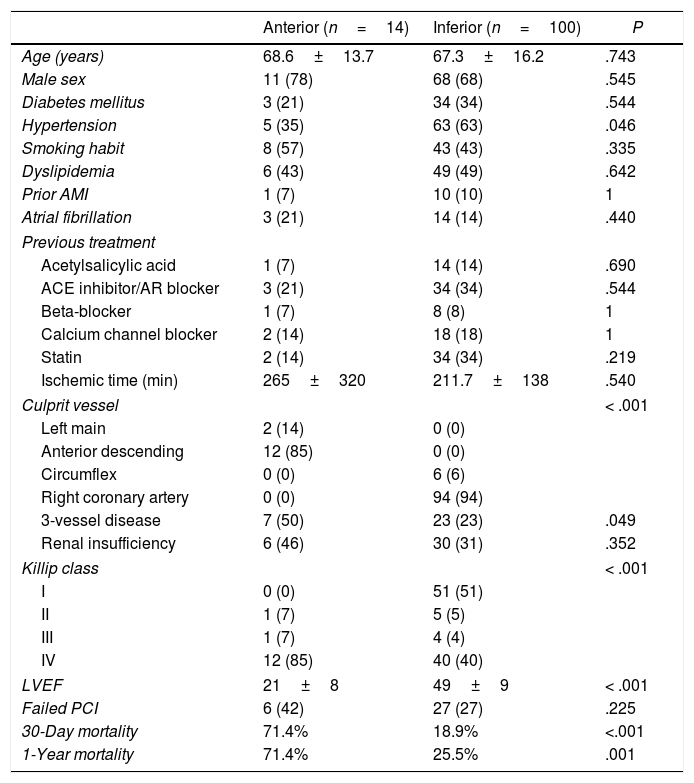
Anterior vs inferior STEMIWe aimed to assess potential differences between patients according to the STEMI location (Table 3). As expected, patients with anterior STEMI showed a worse Killip class and lower LVEF compared to those with inferior STEMI. Thirty-day and 1-year mortality of patients with anterior STEMI were almost 4-fold higher than that of patients with inferior STEMI (Table 3, Fig. 2).
Clinical, echocardiographic and angiographic characteristics according to STEMI location.
| Anterior (n=14) | Inferior (n=100) | P | |
|---|---|---|---|
| Age (years) | 68.6±13.7 | 67.3±16.2 | .743 |
| Male sex | 11 (78) | 68 (68) | .545 |
| Diabetes mellitus | 3 (21) | 34 (34) | .544 |
| Hypertension | 5 (35) | 63 (63) | .046 |
| Smoking habit | 8 (57) | 43 (43) | .335 |
| Dyslipidemia | 6 (43) | 49 (49) | .642 |
| Prior AMI | 1 (7) | 10 (10) | 1 |
| Atrial fibrillation | 3 (21) | 14 (14) | .440 |
| Previous treatment | |||
| Acetylsalicylic acid | 1 (7) | 14 (14) | .690 |
| ACE inhibitor/AR blocker | 3 (21) | 34 (34) | .544 |
| Beta-blocker | 1 (7) | 8 (8) | 1 |
| Calcium channel blocker | 2 (14) | 18 (18) | 1 |
| Statin | 2 (14) | 34 (34) | .219 |
| Ischemic time (min) | 265±320 | 211.7±138 | .540 |
| Culprit vessel | < .001 | ||
| Left main | 2 (14) | 0 (0) | |
| Anterior descending | 12 (85) | 0 (0) | |
| Circumflex | 0 (0) | 6 (6) | |
| Right coronary artery | 0 (0) | 94 (94) | |
| 3-vessel disease | 7 (50) | 23 (23) | .049 |
| Renal insufficiency | 6 (46) | 30 (31) | .352 |
| Killip class | < .001 | ||
| I | 0 (0) | 51 (51) | |
| II | 1 (7) | 5 (5) | |
| III | 1 (7) | 4 (4) | |
| IV | 12 (85) | 40 (40) | |
| LVEF | 21±8 | 49±9 | < .001 |
| Failed PCI | 6 (42) | 27 (27) | .225 |
| 30-Day mortality | 71.4% | 18.9% | <.001 |
| 1-Year mortality | 71.4% | 25.5% | .001 |
AMI, acute myocardial infarction; ACE inhibitor, angiotensin-converting enzyme inhibitor; AR, angiotensin receptor; LVEF, left ventricle ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as no. (%) or mean±standard deviation.
Of the 114 patients included in the analysis, 26 (23%) died during index hospitalization with the TP implanted. In 80 patients (70%) TP was successfully removed and only 8 patients (7%) required permanent pacemaker implantation. Those patients who required permanent pacemaker implantation had higher rates of left bundle branch block (25% vs 2.6%; P=0.044), and longer ischemic times (576 ±761min vs 231±190min; P=.002), compared to those who recovered atrio-ventricular conduction. Time from PPCI to TP retrieval was 1.4±0.7 days, whereas time from PPCI to permanent pacemaker implantation was 5.5±2.3 days. Among patients who required a permanent pacemaker, 2 died within the first 30-days of follow-up, and the remaining 6 were alive after 1-year follow-up.
DiscussionThe present study explores the incidence and outcomes of patients with HAVB who required TP insertion in a large cohort of STEMI patients. The main findings of our study were as follows: (a) patients with STEMI who require TP implantation corresponds to a high-risk population with poor outcomes; (b) LVEF≤40% and acute renal failure are independent mortality risk factors in this patient population; (c) patients with anterior STEMI who required TP had a ∼4-fold increased mortality risk compared to those with inferior STEMI; and (d) TP could be safely removed within the first 24h in most patients.
While the improvement of antithrombotic therapy and early reperfusion have led to a significant decrease in the incidence of HAVB in STEMI patients,7 the rate of TP insertion among this patient population have remained steady between 2.6% and 10.9% in the current PPCI era.8,9 A possible explanation for these wide variations might be the lack of standardization of the optimum time and indication to start temporary pacing between different centers. According to current clinical guidelines, TP should be inserted in patients with STEMI and HAVB with hemodynamic instability not responding to atropine, or with low unstable scape rhythm.10 In our study, this proportion was 3.7%, similar to that reported by Gang et al. in a contemporary cohort.9
Patients in our cohort were highly unstable as shown by the high incidence of cardiogenic shock, ventricular arrhythmias and mortality. They also had extensive coronary artery disease and experienced a high rate of complications during angioplasty such as non-reflow phenomenon or failed-PCI. There was a high 30-day mortality (24.5%) in our study population. This poor outcome is probably not entirely related to the HAVB and temporary pacing itself but also to the relatively larger infarct size, higher reperfusion times and lower rates of reperfusion success. TP insertion in STEMI patients has been previously associated with large infarct size, low LVEF and mortality.7,11,12 Infarct size has been also correlated with development of HAVB and the need for temporary and definitive pacemakers after STEMI.7 Moreover, in a study by Singh et al.,12 TP insertion was associated with an almost 3-fold increase in mortality rate, and proved to be an independent mortality risk factor among patients with HAVB. As in our study, those results suggest that patients with STEMI and HAVB who require TP deserve special consideration and a high degree of skills in the acute phase. Anticipation to the onset of potential complications and a rapid therapeutic response could make a difference on prognosis.
Low LVEF and impaired renal function resulted independently associated with a higher 30-day mortality after multivariate adjustment, both in the entire cohort and in patients with inferior STEMI, where infarct size is usually smaller. When considering only inferior STEMI, where infarct size is usually much lower than in those with anterior STEMI, low LVEF and impaired renal function still remained independently associated with 30-day mortality in the multivariate analysis. Contrarily, right ventricle infarction, a well-known mortality predictor among patients with inferior STEMI,13 did not result an independent mortality risk factor in this model. A potential explanation for this finding is that patients with inferior STEMI and HAVB requiring TP present larger infarct sizes and lower left ventricle function. Thus, the LVEF importance seems to exceed that of the right ventricle infarction in this setting of patients with HAVB who need TP insertion.
Considering STEMI location, although the proportion of patients with anterior STEMI in our study cohort was relatively small, we found that patients with anterior STEMI had almost 4-fold increased mortality risk compared to those with inferior STEMI. Previous studies have reported a dreadful prognosis of heart block in patients with anterior STEMI.14,15 This poorer clinical outcome of HAVB in anterior STEMI compared to inferior MI is due to the different underlying mechanism leading to heart block in each case. While HAVB in inferior STEMI is usually a ‘benign’ process due to increased vagal tone and ischemia of the nodal arteries with rapid recovery after reperfusion, the onset of HAVB in anterior STEMI reflects a vast necrosis that is difficult to recover, even after reperfusion.
Finally, TP could be safely removed within the first 48h in most patients in our cohort. Only 8 of them (7%) required a definitive pacemaker. Patients who needed a permanent pacemaker showed higher rates of left bundle branch block and longer ischemic times compared to those who did not. Current guidelines recommend long waiting times after revascularization before implantation of a definitive pacemaker.10,16 However, the lack of specific recommendation about waiting times has led to open interpretations of the guidelines.3,11,17 Some authors suggest that early permanent pacemaker insertion should be considered in patients with poor or failed revascularization, heart failure, arrhythmias and important comorbidities. However, our results support that a case by case approach should be taken before implantation of a definitive pacemaker.
LimitationsThe present study has certain limitations that should be considered when interpreting the results. Although it includes a very large number of consecutive patients with STEMI with a high quality of data recording, the analysis was retrospective with limitations own to this type of studies. The single center nature of our study limits extrapolation of our results. Our center had an Acute Cardiac Care Unit managed by cardiologists since the start of the study. As this is an important point in management of complex STEMI, such as those developing HAVB requiring TP insertion, our results may not be completely extrapolated to other organizational settings.
As in any registry, under-reporting of TP insertion and clinical events cannot be completely excluded. Temporal variation in the use of antiplatelet agents and other therapies along the wide time span may have influenced the clinical outcomes. Information about bleeding events was not systematically recorded.
Only patients who underwent primary PCI were included in the study. Information about patients who were reperfused with thrombolysis was not recorded. Therefore, our results cannot be applicable to other populations where reperfusion strategy is mainly based on thrombolysis. The current study design does not allow to draw further conclusions on the appropriateness of pacemaker insertion strategy.
There was also a high heterogeneity in the sample as only 14/114 patients had anterior STEMI. However, although the number of patients with anterior MI is small, these patients come from a larger cohort of 3063 consecutive patients with STEMI, 1684 of which had anterior STEMI and only 14 received a temporary pacing. This small number of patients with anterior STEMI limits our statistical power to obtain definitive conclusions on this subgroup. Our results, although limited by the sample size, add contemporary data on the incidence and outcomes of this group of patients.
ConclusionsPatients with STEMI and HAVB who require TP insertion are at great risk of short-term adverse outcomes, especially those with anterior STEMI. Low LVEF and impaired renal function at admission are independently associated with mortality in this patient population. TP could be safely removed in most patients, and only a small proportion of them will require a definitive pacemaker implant.
The improvement of antithrombotic therapy and early reperfusion have led to a significant decrease in the incidence of high-degree atrio-ventricular block in patients with STEMI, the rate of TP insertion among this patient population have remained steady between 2.6% and 10.9% in the current PPCI era.
Does it contribute anything new?- •
The prevalence of temporary pacemaker insertion in a large cohort of patients with STEMI who underwent PPCI was 3.7%.
- •
Patients with STEMI who require temporary pacemaker implantation, particularly those with anterior STEMI, are at great risk of adverse outcome.
- •
Low left ventricle ejection fraction and impaired renal function are independent mortality risk factors in this patient population.
- •
Temporary pacemaker could be safely removed in most patients, and only a small proportion of them will require permanent pacemaker implant.
There is no funding source to declare related to this study.
Authors’ contributionC. Ferrera, L. E. Enríquez, FJ. Noriega, L. Borrego, V. Cañadas, P. Jiménez Quevedo, N. Pérez Castellano, A. Fernández Ortiz, J. Pérez Villacastín, C. Macaya, and A. Viana Tejedor have substantially contributed to the conception of the work. All authors participated in the acquisition, analysis, and interpretation of the data for the work. C. Ferrera and L. E. Enríquez drafted the work. F. J. Noriega, L. Borrego, V. Cañadas, P. Jiménez Quevedo, N. Pérez Castellano, A. Fernández Ortiz, J. Pérez Villacastín, C. Macaya, and A. Viana Tejedor revised it critically for important intellectual content. All authors have given final approval of the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interestThere are no potential conflicts of interest to declare.
Abbreviations: HAVB: high degree atrioventricular block; LVEF: left ventricular ejection fraction; STEMI: ST-segment elevation myocardial infarction; PPCI: primary percutaneous coronary intervention; TP: temporary pacemaker.