Intravenous iron is recommended to correct iron deficiency in heart failure (HF) patients, although not all patients had the same response. We evaluated the impact of ferric carboxymaltose (FCM) in analytical and clinical improvement and analyzed the response according to different parameters.
MethodsWe included HF patients who received FCM in the daily hospital to correct iron deficiency. We recorded clinical and analytical parameters at baseline and 3 months follow-up.
ResultsWe included 236 patients (age 73.5±10.7 years) with left ventricular ejection fraction 44.5±15.3%. There were no adverse effects during the infusion. All ferric analytical parameters significantly increased at 3 months. 25.1% of patients improved functional class, and New York Heart Association (NYHA) III patients were more likely to improve. Mean corpuscular volume predicted NYHA improvement (OR, 0.97; 95%CI, 0.93–1.00; P=.05). Body mass index (OR, 0.86; 95%CI, 0.78–0.95; P=.002) and ferritin (OR, 1.00; 95%CI, 1.00–1.01; P=.042) were also predictors of N-terminal pro-B-type natriuretic peptide reduction of at least 30% from basal levels. 61.3% received lower doses of FCM than the theoretical ones, and those with the correct dose were more likely to improve NYHA and reduce natriuretic peptides.
ConclusionsFCM was useful to correct iron deficiency in clinical practice, independently to anemia, without relevant adverse effects. Clinical improvement was especially relevant in more symptomatic patients and those with absolute deficiency. Mean corpuscular volume, body mass index, ferritin, and dose of FCM were predictors of clinical improvement.
El uso de carboximaltosa férrica (CMF) se recomienda para tratar la ferropenia en pacientes con insuficiencia cardiaca (IC), aunque no todos responden igual. Se analizó el impacto del tratamiento con CMF según diferentes parámetros.
MétodosSe incluyeron los pacientes con IC que recibieron CMF para corregir la ferropenia. Se analizaron diversos parámetros clínicos y analíticos al inicio y a los 3 meses.
ResultadosSe incluyeron 236 pacientes (edad 73,5±10,7años) con fracción de eyección 44,5±15,3%. No hubo efectos adversos durante la infusión. Todos los parámetros férricos aumentaron significativamente. El 25,1% de los pacientes mejoraron la New York Heart Association (NYHA), siendo más frecuente en los NYHA III. El volumen corpuscular medio fue predictor de mejoría de la NYHA (OR=0,97; IC95%, 0,93-1,00). El índice de masa corporal (OR=0,86; IC95%, 0,78-0,95) y la ferritina (OR=1,00; IC95%, 1,00-1,01) fueron predictores de la reducción de los péptidos natriuréticos. El 61,3% de los pacientes recibieron una dosis de CMF menor que la teórica, siendo más frecuente que mejorara la NYHA y se redujeran los péptidos natriuréticos en los que recibieron la dosis correcta.
ConclusionesLa administración de CMF es útil para corregir la ferropenia, independientemente de la anemia, sin efectos adversos. La mejoría clínica fue especialmente relevante en los más sintomáticos y en aquellos con déficit absoluto de hierro. El volumen corpuscular medio, el índice de masa corporal, la ferritina y la dosis de CMF fueron predictores de mejoría.
Iron deficiency (ID) is a common comorbidity in heart failure (HF), regardless of left ventricular ejection fraction (LVEF),1–3 and affects approximately 50% of HF patients.4 ID has been related to impaired clinical status, exercise tolerance, and prognosis.5,6
The evidence for ID correction is based on clinical trials like Ferinject Assessment in Patients With IRon Deficiency and Chronic Heart Failure (FAIR-HF; NCT00520780) and A Study to Compare the Use of Ferric Carboxymaltose With Placebo in Patients With Chronic Heart Failure and Iron Deficiency (CONFIRM-HF; NCT01453608), which demonstrated that ID correction with intravenous (i.v.) iron, specifically ferric carboxymaltose (FCM), reduced HF hospitalization and improved quality of life and symptoms, independently to anemia and renal function.6–8 However, clinical trials included a very different population (anemia, renal function, LVEF, New York Heart Association [NYHA] class, or age), and there is a lack of evidence in some practical issues.
Clinical practice guidelines recommend to periodically study and correct ID due to the high prevalence in HF patients and the impact on prognosis.3,9 However, there are some gaps in practice use, considering the heterogeneity of HF patients and the economic restrictions with FCM. For instance, we do not know if all patients respond in the same way to ID correction, or the preferred tests for diagnosis and follow-up,10 and if there is a relationship between analytical correction and clinical outcomes. This study aims to identify clinical and analytical parameters that can lead the management of HF outpatients with ID and better predict outcomes in real-life clinical practice.
MethodsWe performed an observational registry including all consecutive HF outpatients who received FCM to correct ID between September 2015 and December 2017 in the HF unit of a third level hospital according to European society of cardiology guidelines. The exclusion criteria were patients who refused FCM treatment and not signing the informed consent. We included all-range LVEF patients in our clinical practice routine, due to ID was identified as a relevant parameter in all HF patients,1,2 waiting for future indications derived to the Effect of IV Iron in Patients With Heart Failure With Preserved Ejection Fraction (FAIR-HFpEF; NCT03074591) trial. CFM had been used in different patients’ profiles to correct ID without adverse effects, even in patients without heart disease.11 The study complied with the Declaration of Helsinki and was approved by the hospitals' Ethics Committees. All patients agreed with the possibility of using their data for research purposes and signed the informed consent.
ID was defined as ferritin<100ng/mL (absolute deficiency) or ferritin 100–300ng/mL with transferrin saturation (TSAT)<20% (functional deficiency) according to guidelines.9,12 Anemia was defined as hemoglobin (Hb)<12g/dL (female) and <13g/dL (men).3,9 The i.v. dose of FCM was calculated based on weight and Hb3: Hb 10–14g/dL and weight<70kg: 1000mg; Hb<10g/dL and weight<70kg: 1500mg; Hb≥10g/dL and <14h/dL and weight≥70kg: 1500mg; Hb<10g/dL and weight≥70kg: 2000mg; Hb≥14g/dL: 500mg. FCM was administred in saline 0.9% (50–150mL) in 15min (maximum 1000mg of FCM a week) in the daily hospital with nurse supervision and 30min monitoring post-infusion.
Different variables were analyzed: (a) medical history, vital signs, NYHA functional class, LVEF, previous treatment; (b) blood test parameters: hematic and ferric parameters, N-terminal pro-B-type natriuretic peptide (NT-proBNP), renal function, and electrolytes; (c) aspects related to FCM: dose and adverse effects; d) functional tests: health-related quality of life (HRQoL) with EQ-5D-3L test (Spanish version 2014)13 and the Spanish version of Minnesota Living with Heart Failure Questionnaire (MLHFQ).14,15 Clinical and analytical parameters were repeated at 3 months, according to clinical practice guidelines.3,16 In case of ID recurrence (defined as ferritin<100ng/mL or ferritin 100–300ng/mL with TSAT<20%) a new FCM infusion was administrated in the next few days.
Quantitative variables are expressed as mean±standard deviation or median and interquartile range in nonparametric data, and qualitative variables as number and percentage. The Student t test or the sum of Wilcoxon ranges in nonparametric data was used to compare continuous quantitative variables. Categorical variables were compared with the chi-square test and Fisher's exact test. A significance level of 0.05 (bilateral) was established for all statistical tests. We performed an analysis according to basal TSAT, NYHA class, anemia status, and FCM dose.
Logistic regression was performed to identify predictors of: (a) NYHA functional improvement; (b) prognosis improvement with NT-proBNP reduction of at least 30% from basal, according to the cut-off established in other studies.17 NYHA improvement was defined as a change in NYHA class determined by medical criteria. The multivariate analysis included: age, sex, basal LVEF, body mass index, basal NYHA, NYHA improvement, NT-proBNP, albumin, basal ferritin, basal hemoglobin, basal TSAT, basal mean corpuscular volume, basal serum iron, basal mean corpuscular hemoglobin concentration, basal estimated glomerular filtration rate, FCM dose, and the absolute difference between theoretical and administered dose of FCM.
It was performed a linear regression to study the parameters related to TSAT increase, as it is considered better than other markers (i.e., ferritin) to study body iron status and had been linked to cardiovascular mortality.18,19 A significance level of 0.05 (bilateral) was established for all statistical tests. The statistical analysis was performed with Stata 13.1 and SPSS 21.0.
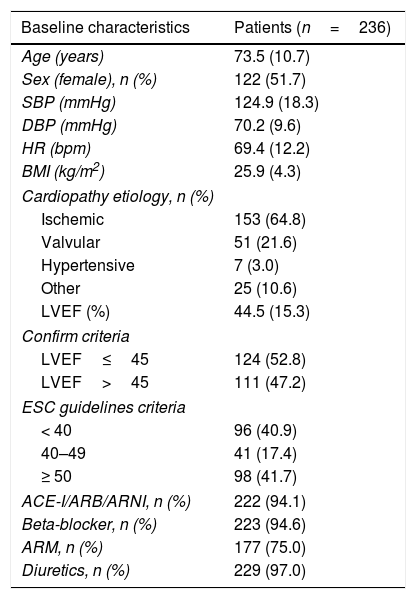
ResultsWe included 236 patients with a mean age of 73.5±10.7 years (51.7% female). Baseline characteristics are attached in Table 1. All patients received optimal treatment for HF and were optimized during the follow-up. Mean FCM dose was 947.0±154.2mg (median 1000mg). One patient presented a local exanthema resolved with oral antihistaminics and corticosteroids. There were no other adverse effects. Analytical and clinical parameters are summarized in Table 2.
Baseline characteristics of patients with FCM administration.
| Baseline characteristics | Patients (n=236) |
|---|---|
| Age (years) | 73.5 (10.7) |
| Sex (female), n (%) | 122 (51.7) |
| SBP (mmHg) | 124.9 (18.3) |
| DBP (mmHg) | 70.2 (9.6) |
| HR (bpm) | 69.4 (12.2) |
| BMI (kg/m2) | 25.9 (4.3) |
| Cardiopathy etiology, n (%) | |
| Ischemic | 153 (64.8) |
| Valvular | 51 (21.6) |
| Hypertensive | 7 (3.0) |
| Other | 25 (10.6) |
| LVEF (%) | 44.5 (15.3) |
| Confirm criteria | |
| LVEF≤45 | 124 (52.8) |
| LVEF>45 | 111 (47.2) |
| ESC guidelines criteria | |
| < 40 | 96 (40.9) |
| 40–49 | 41 (17.4) |
| ≥ 50 | 98 (41.7) |
| ACE-I/ARB/ARNI, n (%) | 222 (94.1) |
| Beta-blocker, n (%) | 223 (94.6) |
| ARM, n (%) | 177 (75.0) |
| Diuretics, n (%) | 229 (97.0) |
ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARM, mineralocorticoid receptor antagonists; ARNI, angiotensin receptor antagonist and neprilysin inhibitor; BMI, body mass index; BPM, beats per minute; DBP, diastolic blood pressure; ESC, European Society of Cardiology; FCM, ferric carboxymaltose; HR, heart rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure.
CONFIRM criteria refer to LVEF inclusion in the CONFIRM-HF trial (≤ 45%)6. ESC guidelines criteria involve reduced, mid-range, and preserved ejection fraction9.
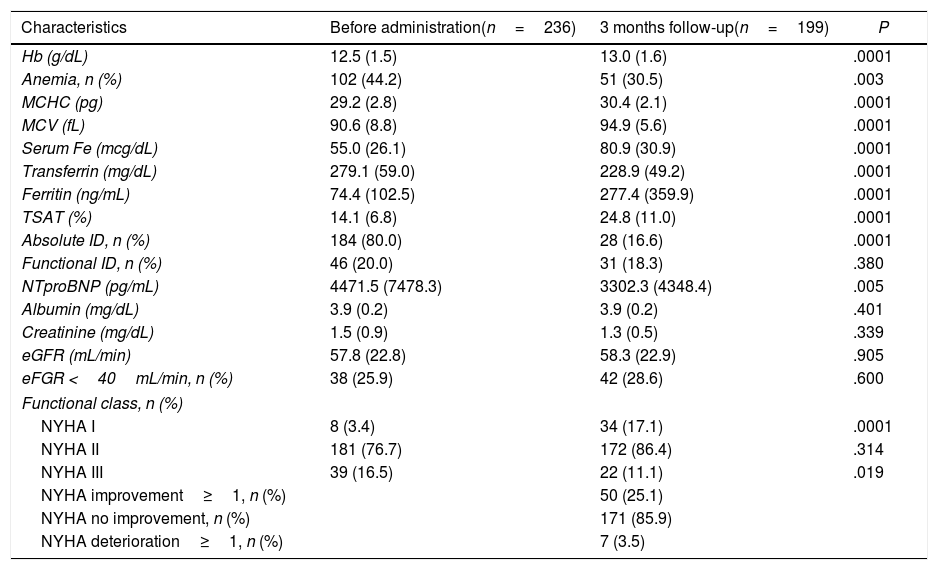
Comparative of characteristics of the patients with FCM infusion at three months.
| Characteristics | Before administration(n=236) | 3 months follow-up(n=199) | P |
|---|---|---|---|
| Hb (g/dL) | 12.5 (1.5) | 13.0 (1.6) | .0001 |
| Anemia, n (%) | 102 (44.2) | 51 (30.5) | .003 |
| MCHC (pg) | 29.2 (2.8) | 30.4 (2.1) | .0001 |
| MCV (fL) | 90.6 (8.8) | 94.9 (5.6) | .0001 |
| Serum Fe (mcg/dL) | 55.0 (26.1) | 80.9 (30.9) | .0001 |
| Transferrin (mg/dL) | 279.1 (59.0) | 228.9 (49.2) | .0001 |
| Ferritin (ng/mL) | 74.4 (102.5) | 277.4 (359.9) | .0001 |
| TSAT (%) | 14.1 (6.8) | 24.8 (11.0) | .0001 |
| Absolute ID, n (%) | 184 (80.0) | 28 (16.6) | .0001 |
| Functional ID, n (%) | 46 (20.0) | 31 (18.3) | .380 |
| NTproBNP (pg/mL) | 4471.5 (7478.3) | 3302.3 (4348.4) | .005 |
| Albumin (mg/dL) | 3.9 (0.2) | 3.9 (0.2) | .401 |
| Creatinine (mg/dL) | 1.5 (0.9) | 1.3 (0.5) | .339 |
| eGFR (mL/min) | 57.8 (22.8) | 58.3 (22.9) | .905 |
| eFGR <40mL/min, n (%) | 38 (25.9) | 42 (28.6) | .600 |
| Functional class, n (%) | |||
| NYHA I | 8 (3.4) | 34 (17.1) | .0001 |
| NYHA II | 181 (76.7) | 172 (86.4) | .314 |
| NYHA III | 39 (16.5) | 22 (11.1) | .019 |
| NYHA improvement≥1, n (%) | 50 (25.1) | ||
| NYHA no improvement, n (%) | 171 (85.9) | ||
| NYHA deterioration≥1, n (%) | 7 (3.5) | ||
eGFR, estimated glomerular filtrated rate; FCM, ferric carboxymaltose; Fe, Iron; Hb, hemoglobin; ID, iron deficiency; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; NYHA, New York Heart Association; TSAT, transferrin saturation.
Functional or absolute ID are defined according to ferritin and TSAT3.
The analytic control at three months follow-up was performed in all alive patients.
A total of 162 patients (68.6%) reported clinical improvement after FCM administration. If we consider NYHA class, 171 patients (85.9%) did not change functional status, and 50 (25.1%) improved (Table 2). The comparative of HRQoL tests is attached in the table 1 of the supplementary data.
Patients with basal NYHA II received lower doses of FCM compared to NYHA III (947.5±153.7 vs 961.5±135.0mg; P=.044). NYHA III patients referred more frequently clinical improvement (71.8% vs 67.4%; P=.04) and were more likely to improve functional class at 3 months than NYHA II (56.6% vs 15.5%; P=.0001). There were not basal differences in clinical or analytical parameters according to basal NYHA, and the rate of absolute ID was similar (NYHA II 80.9% vs NYHA III 78.9%; P=.577).
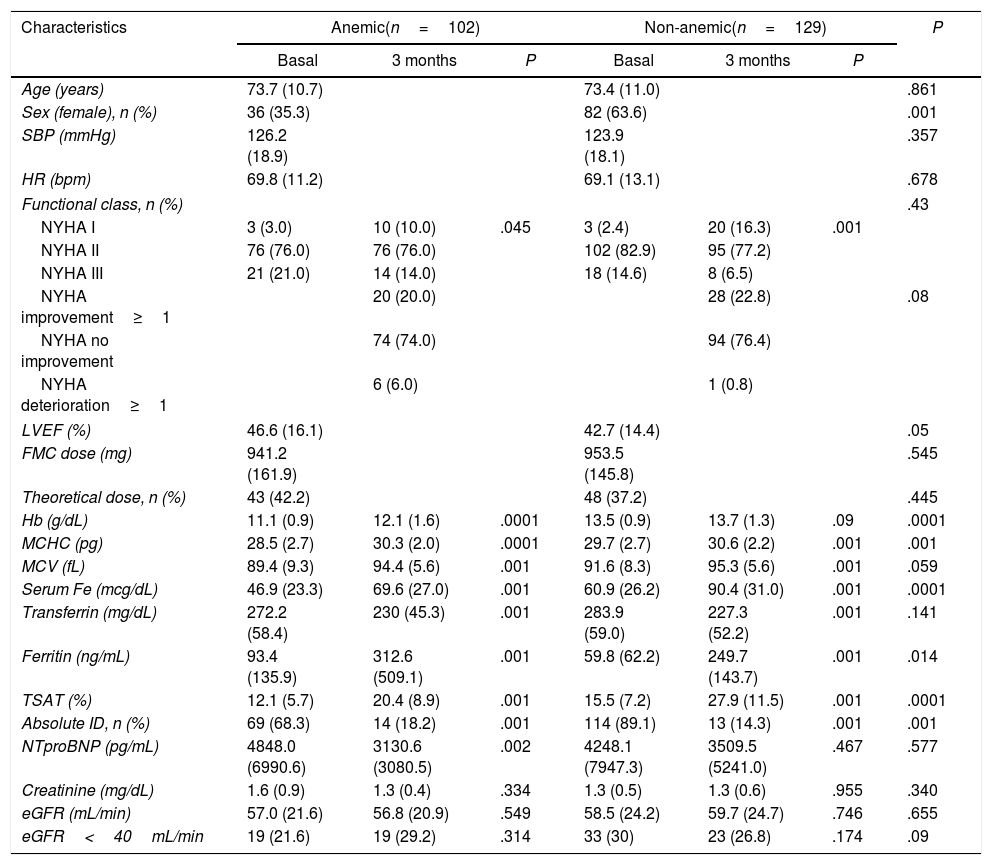
At 3 months, 41.6% of anemic patients normalized Hb levels (P=.0001), as it is shown in Table 3. The 6.7% of non-anemic patients developed anemia during the follow-up. Also, 17.5% of patients with basal TSAT<20% normalized Hb levels (P=.003) and 65% normalized TSAT (P=.0001) during the follow-up (table 2 of the supplementary data).
Comparative of the characteristics of the patients at three months according to the presence of anemia.
| Characteristics | Anemic(n=102) | Non-anemic(n=129) | P | ||||
|---|---|---|---|---|---|---|---|
| Basal | 3 months | P | Basal | 3 months | P | ||
| Age (years) | 73.7 (10.7) | 73.4 (11.0) | .861 | ||||
| Sex (female), n (%) | 36 (35.3) | 82 (63.6) | .001 | ||||
| SBP (mmHg) | 126.2 (18.9) | 123.9 (18.1) | .357 | ||||
| HR (bpm) | 69.8 (11.2) | 69.1 (13.1) | .678 | ||||
| Functional class, n (%) | .43 | ||||||
| NYHA I | 3 (3.0) | 10 (10.0) | .045 | 3 (2.4) | 20 (16.3) | .001 | |
| NYHA II | 76 (76.0) | 76 (76.0) | 102 (82.9) | 95 (77.2) | |||
| NYHA III | 21 (21.0) | 14 (14.0) | 18 (14.6) | 8 (6.5) | |||
| NYHA improvement≥1 | 20 (20.0) | 28 (22.8) | .08 | ||||
| NYHA no improvement | 74 (74.0) | 94 (76.4) | |||||
| NYHA deterioration≥1 | 6 (6.0) | 1 (0.8) | |||||
| LVEF (%) | 46.6 (16.1) | 42.7 (14.4) | .05 | ||||
| FMC dose (mg) | 941.2 (161.9) | 953.5 (145.8) | .545 | ||||
| Theoretical dose, n (%) | 43 (42.2) | 48 (37.2) | .445 | ||||
| Hb (g/dL) | 11.1 (0.9) | 12.1 (1.6) | .0001 | 13.5 (0.9) | 13.7 (1.3) | .09 | .0001 |
| MCHC (pg) | 28.5 (2.7) | 30.3 (2.0) | .0001 | 29.7 (2.7) | 30.6 (2.2) | .001 | .001 |
| MCV (fL) | 89.4 (9.3) | 94.4 (5.6) | .001 | 91.6 (8.3) | 95.3 (5.6) | .001 | .059 |
| Serum Fe (mcg/dL) | 46.9 (23.3) | 69.6 (27.0) | .001 | 60.9 (26.2) | 90.4 (31.0) | .001 | .0001 |
| Transferrin (mg/dL) | 272.2 (58.4) | 230 (45.3) | .001 | 283.9 (59.0) | 227.3 (52.2) | .001 | .141 |
| Ferritin (ng/mL) | 93.4 (135.9) | 312.6 (509.1) | .001 | 59.8 (62.2) | 249.7 (143.7) | .001 | .014 |
| TSAT (%) | 12.1 (5.7) | 20.4 (8.9) | .001 | 15.5 (7.2) | 27.9 (11.5) | .001 | .0001 |
| Absolute ID, n (%) | 69 (68.3) | 14 (18.2) | .001 | 114 (89.1) | 13 (14.3) | .001 | .001 |
| NTproBNP (pg/mL) | 4848.0 (6990.6) | 3130.6 (3080.5) | .002 | 4248.1 (7947.3) | 3509.5 (5241.0) | .467 | .577 |
| Creatinine (mg/dL) | 1.6 (0.9) | 1.3 (0.4) | .334 | 1.3 (0.5) | 1.3 (0.6) | .955 | .340 |
| eGFR (mL/min) | 57.0 (21.6) | 56.8 (20.9) | .549 | 58.5 (24.2) | 59.7 (24.7) | .746 | .655 |
| eGFR<40mL/min | 19 (21.6) | 19 (29.2) | .314 | 33 (30) | 23 (26.8) | .174 | .09 |
BMI, body mass index; BPM, beats per minute; DBP, diastolic blood pressure; eGFR, estimated glomerular filtrated rate; FCM, ferric carboxymaltose; Fe, Iron; Hb, hemoglobin; HR, heart rate; ID, iron deficiency; LVEF, left ventricular ejection fraction; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; SBP, systolic blood pressure; TSAT, transferrin saturation.
5 patients were excluded from the analysis for incomplete data.
The correct dose was defined according to weight and Hb3. Functional ID was defined as ferritin<1003.
Mean corpuscular volume was identified as an independent predictor of NYHA improvement (odds ratio [OR], 0.97; 95% confidence interval [95%CI], 0.93–1.00; P=.045). Body mass index (OR, 0.86; 95%CI, 0.78–0.95; P=.002) and ferritin levels (OR, 1.00; 95%CI, 1.00–1.01; P=.042) were also identified as predictors of NT-proBNP reduction. Also, the increase of one unit of basal serum iron reduced TSAT levels in 0.25% (P=.001), and the increase of one unit of basal Hb increased TSAT levels in 2.91% (P=.001).
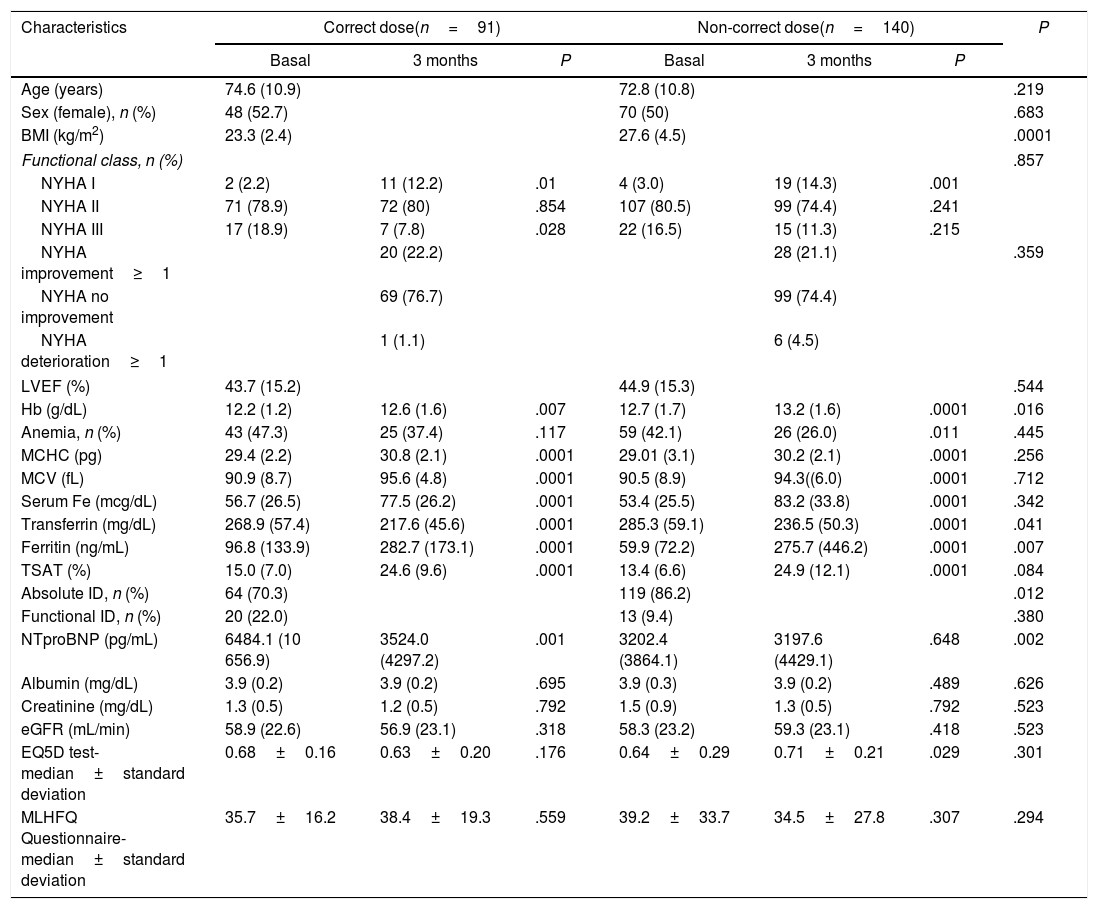
Ninety patients (38.7%) received the correct dose (mean 989.0±73.7mg), and 140 (61.3%) the non-correct dose (mean 921.4±182.6mg; P=.001), with a difference with the theoretical dose of −282.1±484.3mg (Table 4). 22.2% of patients with correct doses improved NYHA class and 21.2% of those with non-correct dose (P=.359). After 3 months, 22% of patients recurred and received a new infusion. It was observed an NT-proBNP reduction in 52.9% of patients with the correct dose and 34% of patients with non-correct dose (P=.015). There was a deterioration in HRQoL tests at 3 months in patients with non-correct dose in question number 6 (2.23±1.56 vs 1.29±1.37; P=.008) and 8 (1.85±2.2 vs 1.04±1.59; P=.026) of the Minnesota test. Also, there was an improvement in patients administered a correct dose in question 20 (2.13±1.54 vs 1.00±1.21; P=.031) and 21 (2.0±1.59 vs 0.81±1.11; P=.015) in the Minnesota test.
Comparative of the characteristics of the patients at three months according to FCM infusion dose.
| Characteristics | Correct dose(n=91) | Non-correct dose(n=140) | P | ||||
|---|---|---|---|---|---|---|---|
| Basal | 3 months | P | Basal | 3 months | P | ||
| Age (years) | 74.6 (10.9) | 72.8 (10.8) | .219 | ||||
| Sex (female), n (%) | 48 (52.7) | 70 (50) | .683 | ||||
| BMI (kg/m2) | 23.3 (2.4) | 27.6 (4.5) | .0001 | ||||
| Functional class, n (%) | .857 | ||||||
| NYHA I | 2 (2.2) | 11 (12.2) | .01 | 4 (3.0) | 19 (14.3) | .001 | |
| NYHA II | 71 (78.9) | 72 (80) | .854 | 107 (80.5) | 99 (74.4) | .241 | |
| NYHA III | 17 (18.9) | 7 (7.8) | .028 | 22 (16.5) | 15 (11.3) | .215 | |
| NYHA improvement≥1 | 20 (22.2) | 28 (21.1) | .359 | ||||
| NYHA no improvement | 69 (76.7) | 99 (74.4) | |||||
| NYHA deterioration≥1 | 1 (1.1) | 6 (4.5) | |||||
| LVEF (%) | 43.7 (15.2) | 44.9 (15.3) | .544 | ||||
| Hb (g/dL) | 12.2 (1.2) | 12.6 (1.6) | .007 | 12.7 (1.7) | 13.2 (1.6) | .0001 | .016 |
| Anemia, n (%) | 43 (47.3) | 25 (37.4) | .117 | 59 (42.1) | 26 (26.0) | .011 | .445 |
| MCHC (pg) | 29.4 (2.2) | 30.8 (2.1) | .0001 | 29.01 (3.1) | 30.2 (2.1) | .0001 | .256 |
| MCV (fL) | 90.9 (8.7) | 95.6 (4.8) | .0001 | 90.5 (8.9) | 94.3((6.0) | .0001 | .712 |
| Serum Fe (mcg/dL) | 56.7 (26.5) | 77.5 (26.2) | .0001 | 53.4 (25.5) | 83.2 (33.8) | .0001 | .342 |
| Transferrin (mg/dL) | 268.9 (57.4) | 217.6 (45.6) | .0001 | 285.3 (59.1) | 236.5 (50.3) | .0001 | .041 |
| Ferritin (ng/mL) | 96.8 (133.9) | 282.7 (173.1) | .0001 | 59.9 (72.2) | 275.7 (446.2) | .0001 | .007 |
| TSAT (%) | 15.0 (7.0) | 24.6 (9.6) | .0001 | 13.4 (6.6) | 24.9 (12.1) | .0001 | .084 |
| Absolute ID, n (%) | 64 (70.3) | 119 (86.2) | .012 | ||||
| Functional ID, n (%) | 20 (22.0) | 13 (9.4) | .380 | ||||
| NTproBNP (pg/mL) | 6484.1 (10 656.9) | 3524.0 (4297.2) | .001 | 3202.4 (3864.1) | 3197.6 (4429.1) | .648 | .002 |
| Albumin (mg/dL) | 3.9 (0.2) | 3.9 (0.2) | .695 | 3.9 (0.3) | 3.9 (0.2) | .489 | .626 |
| Creatinine (mg/dL) | 1.3 (0.5) | 1.2 (0.5) | .792 | 1.5 (0.9) | 1.3 (0.5) | .792 | .523 |
| eGFR (mL/min) | 58.9 (22.6) | 56.9 (23.1) | .318 | 58.3 (23.2) | 59.3 (23.1) | .418 | .523 |
| EQ5D test-median±standard deviation | 0.68±0.16 | 0.63±0.20 | .176 | 0.64±0.29 | 0.71±0.21 | .029 | .301 |
| MLHFQ Questionnaire-median±standard deviation | 35.7±16.2 | 38.4±19.3 | .559 | 39.2±33.7 | 34.5±27.8 | .307 | .294 |
BMI, body mass index; BPM, beats per minute; DBP, diastolic blood pressure; eGFR, estimated glomerular filtrated rate; EQ5D, EuroQOL five dimensions questionnaire; FCM, ferric carboxymaltose; Fe, Iron; Hb, hemoglobin; HR, heart rate; ID, iron deficiency; LVEF, left ventricular ejection fraction; MCHC, mean corpuscular hemoglobin concentration; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MCV, mean corpuscular volume; SBP, systolic blood pressure; TSAT, transferrin saturation.
The correct or noncorrect doses were defined according to weight and Hb3.
Five patients were excluded from the analysis for incomplete data.
EQ5D test and MLHFQ Questionnaire refers to mean punctuation in the global score.
To the best of our knowledge, this is one of the first studies evaluating predictors of functional and analytical improvement in a real-world cohort of HF patients with ID, especially considering the importance of the dose of FCM. They improved analytic parameters, including NT-proBNP, without relevant adverse effects. Clinical improvement was especially relevant in more symptomatic patients and those with absolute deficiency. Mean corpuscular volume, body mass index, ferritin, and the dose of FCM were identified as predictors of improvement.
Our patients were slightly older, with more comorbidities and less symptomatic than those included in other studies.7,20–22 We considered all-range LVEF, though the evidence is more robust in those with reduced LVEF, some studies included ID patients with preserved LVEF, and they had diminished exercise capacity and HRQoL.22,23
FCM administration was safe, similar to other studies,6–8,22 and improved analytic parameters, including NT-proBNP, although the change was especially marked in patients with absolute ID or anemia. Body mass index, and especially ferritin, linked to inflammation status,24 may be useful in clinical practice as they were identified as predictors of NT-proBNP decrease. Toblli et al.25 and Zhou et al.26 had previously observed an N-proBNP reduction in HF patients with ID treated with i.v. iron therapy independently to anemia, but they did not establish a specific NT-proBNP reduction to impact prognosis as we did. Also, TSAT could be one of the most reliable parameters to study ID,19 as it was linked to Hb changes, probably identifying iron status repletion. Patients with lower TSAT and those with anemia benefit the most of i.v. iron administration in our study. Previously, Grote Beverborg et al., in an analysis of DEFINE-HF and BIOSTAT-CHF trials, showed that only absolute ID was related to prognosis,27 so that we need more studies considering this aspect to make specific recommendations for both functional and absolute ID patients.
Nearly 70% of our patients reported functional improvement after FCM, but 85.9% of patients did not objectively change NYHA class or results in HRQoL tests, although NYHA improvement was more likely in more symptomatic patients. Some studies showed NYHA improvement with FCM. In a meta-analysis, Kapoor et al., including patients with anemia, showed a mean reduction of NYHA of 1.2 classes.8 Zhou et al., in another meta-analysis with 1404 patients, described the heterogenicity of NYHA and HRQoL scores in different studies, with the impossibility of control them even with a sensitivity analysis.26 In FAIR-HF, HRQoL tests partially improved at 4 weeks, but they did not at 3 months, similar to our results.20 Functional improvement is linked to several factors, such as etiology and stage of HF (i.e., 21.6% of our patients had valvular HF), ID etiology, renal function,6,24 exercise tolerance,28 and dose of FCM,21 so it is difficult to objective functional changes, especially in elderly patients. Mean corpuscular volume may help identify patients more likely to improve NYHA, as it was a relevant parameter in ID identification and HF prognosis,3,29,30 and was also identified in our study as an NYHA improvement predictor. We need to progressively incorporate other tests to objective these clinical changes, such as the six-minute walk test or maximal oxygen consumption.
In our study, half of the patients received the correct dose, and NYHA II was more unlikely to receive the correct dose, probably because they were wrongly considered as lower risk than NYHA III. The importance of FCM dose in prognosis was explored by Martens et al. in a study with 211 patients with HF and reduced LVEF, with less functional and analytical improvement in non-correct dose.21 FCM correct dose impacted the quality of life, improving depression and concentration items in HRQoL tests in our cohort, similar to FAIR-HF results.20 The reasons for using lower doses in clinical practice were economic restrictions and avoiding several visits to the hospital for iron administration, as it was recommended to check and correct ferric status at 12, 24, and 36 weeks after the first infusion.6,16 However, FCM is cost-effective3 and reduced hospital visits compared to other i.v. iron molecules (i.e., iron sucrose), with a maximum of two visits for FCM injections in 75% of ptients.6
LimitationsOur study had some limitations. First of all, there were some missing visits or incomplete data collection during follow-up. Secondly, we had some logistic and economic limitations to administrate the theoretical dose, which can reduce the final benefit, and it was analyzed in this study. Secondly, the election of a 30% reduction in NT-proBNP could explain the results, but we thought that, according to other studies, other cuts had less impact. Thirdly, longer follow-up could have shown better clinical outcomes, but current clinical guidelines recommend reevaluating these patients at 3 months. Finally, we did not analyze the influence of treatment optimization in functional outcomes, and we did not consider other events like HF readmission or mortality.
ConclusionsIn conclusion, mean corpuscular volume, body mass index, ferritin, and the dose of FCM have been identified as predictors of NYHA or NT-proBNP improvement in HF patients with ID. The administration of FCM in clinical practice is safe and improves all analytical parameters, including natriuretic peptides. Also, the increase of functional status is more likely in the most symptomatic patients, in those with ID absolute deficiency and when the correct dose of FCM is administered.
ID correction with FCM reduces hospital admission and improves clinical status in heart failure patients, but there are some gaps in evidence about some practical issues in real-word clinical practice.
Does it contribute anything new?FCM administration in HF patients with ID improves analytical parameters, without adverse effects. Clinical improvement was more relevant in symptomatic patients and those with absolute deficiency. Mean corpuscular volume, body mass index, ferritin, and dose of FCM were predictors of improvement.
Vifor Pharma had an economic contribution to statistical analysis performance.
Conflicts of interestA. Esteban-Fernández, M. Méndez-Bailón, M. Pérez-Serrano and R. Bover-Freire received payments for scientific conferences by Vifor Pharma. F. Tornero-Molina, M. González-Barja, F.J. Martín-Sánchez and C. Ramírez-Ramos indicate no conflicts of interest.
Abbreviations: FCM: ferric carboximaltose; HF: heart failure; ID: iron deficiency; HRQoL: health-related quality of life; NYHA: New York Heart Association; TSAT: transferrin saturation.