Cardiac contractility modulation (CCM) is one of the latest developed therapies aimed for patients with heart failure. Heart failure is a highly prevalent disease with a poor prognosis. Cardiac resynchronization therapy (CRT) has proved to reduce morbidity and mortality 1in patients with reduced left ventricle ejection fraction (LVEF) and QRS>150ms and improve symptoms in patients with QRS>130ms. However, there is still a high percentage of patients who do not match these criteria and could benefit from other treatment options. The CCM device implanting procedure is similar to a pacemaker placement. It consists of positioning two bipolar leads at the right ventricular septum (local sense and right ventricle) that deliver non-activating biphasic high voltage impulses (generally 7.5V at 22ms) during the absolute refractory period. The use of this device has already been approved for patients with New York Heart Association (NYHA) class III heart failure and LVEF between 25% and 45% who remain symptomatic despite optimized medical treatment and who do not meet criteria for CRT. Four randomized clinical trials have shown benefits in exercise capacity and quality of life though long-term outcome data are still lacking.2,3 Many of these patients are also suitable for implantable cardioverter-defibrillator (ICD).4 At the moment, a device that combines CCM and ICD has not yet been developed, therefore these patients would need to carry 2 different devices, both on the right and left side, requiring at least 3 transvenous leads. Subcutaneous ICD (S-ICD) is an alternative to transvenous ICD that could be particularly useful in these cases, reducing the risk of lead-related complications and allowing us to keep one of the superior venous accesses free, so that it is available in case of need. Röger et al. published their long-term results of 20 patients treated with the combination of these two devices, showing successful outcomes with no problematic device interaction.5
We present the first 2 cases who underwent combined CCM and S-ICD therapy in our hospital. Patients’ written informed consents were obtained.
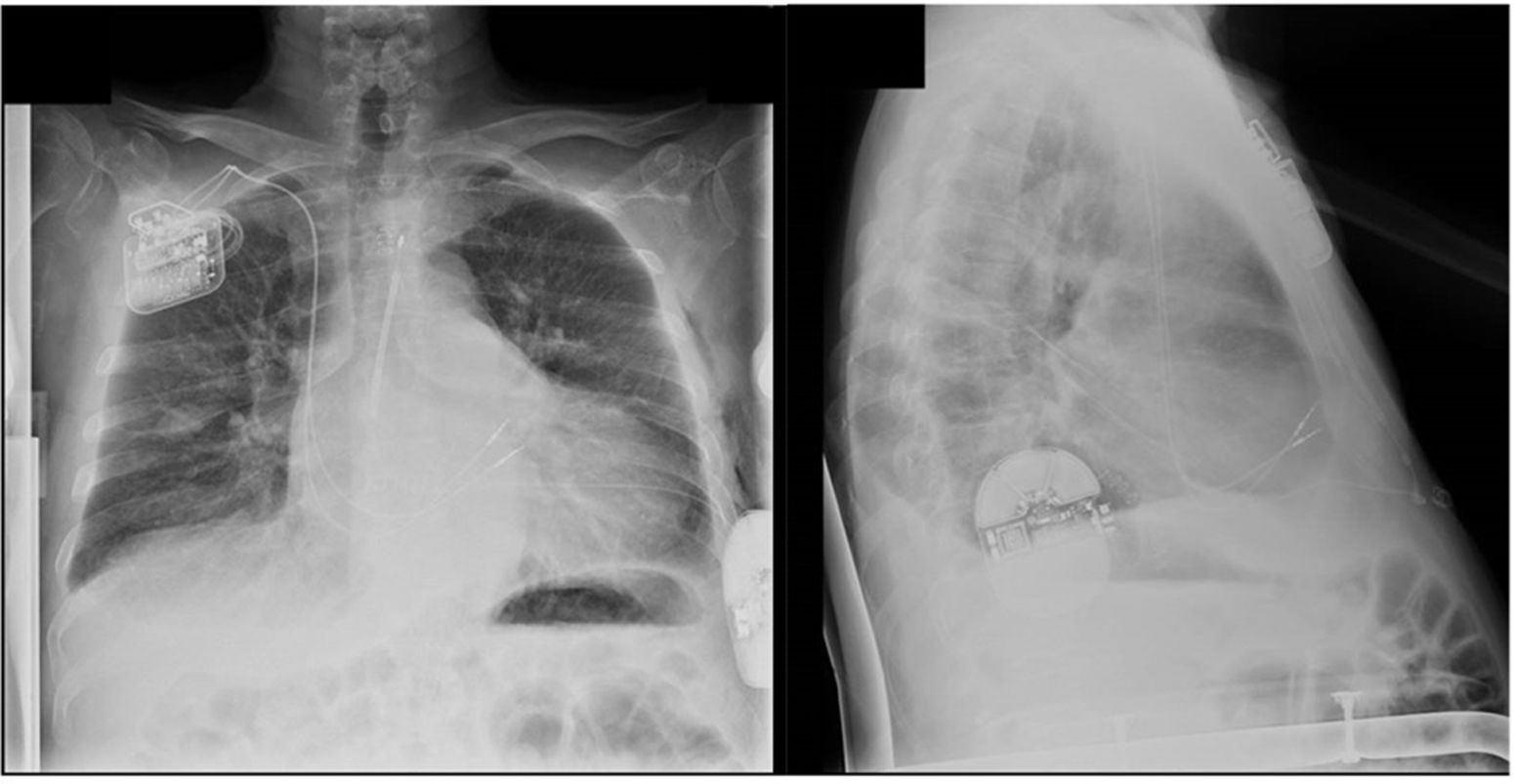
The first patient is a 76-year-old woman with ischemic cardiomyopathy with LVEF of 31%, a NYHA class III-IV heart failure despite optimal medical treatment and a very limited functional capacity with a respiratory exchange ratio of 1.15 and a peak oxygen consumption (VO2) of 8mL/kg/min (Weber: D, Ventilatory Class: IV). The electrocardiogram showed a narrow QRS, so she was not suitable for CRT therapy. Therefore, a CCM Optimizer (Impulse Dynamics, USA) device was implanted. Afterwards, a S-ICD screening was performed both with CCM pacing on and off, with a positive test for the secondary vector in both right and left parasternal positions. Hence, the device was implanted using the 3 incisions technique (Fig. 1). We checked correct S-ICD sensing with CCM pacing activated and performed a successful defibrillation test. At the 6-month-follow-up visit, the patient referred a NYHA II functional class, both devices worked correctly, and she had received no shocks. A new ergospirometry was performed, demonstrating significant functional capacity improvement (Weber: C, Ventilatory class: III). VO2 increased from 8 to 10mL/kg/min and respiratory exchange ratio improved from 1.15 to 1.25. There was also an increase in ventilatory equivalent for oxygen (VEVO2), from 55 to 41, and in the oxygen uptake efficiency slope, which rose from 0.87 to 1.1.
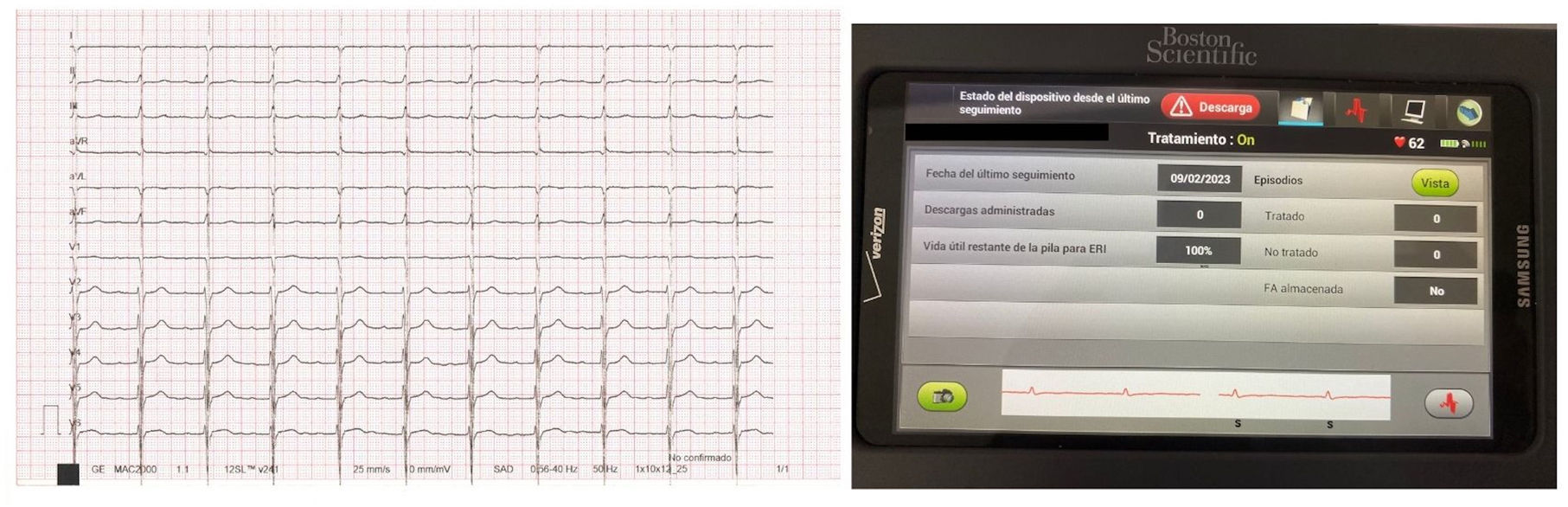
The second patient is a 75-year-old male who also suffers from ischemic cardiomyopathy with a NYHA III functional class and a LVEF of 29%, severe pulmonary hypertension, persistent atrial fibrillation and a narrow QRS complex. In December 2022 an Impulse Dynamics Optimizer CCM was implanted. One month later, after a positive screening for the 3 vectors, a S-ICD was also implanted. During the second procedure a crosstalk test was performed. At the beginning, the S-ICD detected as noise some of the QRS complexes during CCM pacing at 7.5V in all the different vector configurations. We recognized that the CCM was programmed with a very short delay between local sense and right ventricle stimulation. After reprogramming the device with a 45ms delay the problem was solved and no further noise was detected (Fig. 2).
Our initial experience suggests that the combination of CCM and S-ICD is a safe strategy provided that a crosstalk test is performed during the implant procedure to rule out device interference and adjust the configuration if necessary.
This manuscript was conducted in accordance with the Declaration of Helsinki and meets the requirements for manuscripts submitted to biomedical journals of the ICMJE.
FundingNone.
Authors’ contributionsL. Valverde Soria, L. Jordán-Martínez and F. Bermúdez, collected and analyzed the data. L. Valverde Soria wrote the manuscript with the help of the other authors. E. Cabrera Borrego, M. Molina-Lerma and M. Álvarez performed and supervised the technical procedures. M. Álvarez and M. Molina-Lerma conceived the idea and supervised the overall process. All authors discussed the results and contributed to the final manuscript.
Conflicts of interestM. Álvarez has participated on a Boston Scientific (Abott) Advisory Board. The rest of the authors have no conflicts of interest.