Primary care is the gateway of individuals in healthcare systems. Natriuretic peptides measurement is a simple and accessible technique that can be safely used to identify composite outcomes. The main aim of the current study was to assess B-type natriuretic peptides (BNP) performance as prognostic marker in primary care patients with and without heart failure.
MethodsAn observational, retrospective study, deriving from the DIGITALIS study, was conducted with 560 individuals ≥ 45 years old (63.4% women), who had completed 5-year follow-up. Patients were assessed to identify cardiovascular risk factors and the incidence or not of heart failure. Laboratory test, electrocardiogram, and tissue Doppler echocardiography were performed.
ResultsComposite outcome of-all-cause death or hospitalization due to cardiovascular disease was observed for 11.4% of individuals (53.1% women). After multivariable cox regression analysis to minimize the effects of confounding variables, BNP maintained statistical significance. The area under the ROC curve recorded for BNP to identify composite outcome was 0.75 (95%CI, 0.672–0.817; P<.0001). BNP cut-off value of 25pg/mL was the best correlated with composite outcome (sensitivity: 72%, specificity: 71%, accuracy: 91%, negative predictive value: 95%, positive likelihood ratio: 24%).
ConclusionsThe study has shown that BNP is a death predictor in primary care patients with or without heart failure.
La atención primaria es la puerta de entrada de los pacientes a los sistemas sanitarios. La medición de péptidos natriuréticos es una técnica sencilla y accesible que se puede utilizar de forma segura para identificar resultados compuestos. El objetivo principal del estudio actual fue evaluar el rendimiento de los péptidos natriuréticos de tipoB (BNP) como marcador pronóstico en pacientes de atención primaria con y sin insuficiencia cardiaca.
MétodosEstudio observacional, retrospectivo, derivado del estudio DIGITALIS, realizado con 560 individuos (63,4% mujeres), de 45años o más, que habían completado 5años de seguimiento. Se evaluó a los pacientes para identificar factores de riesgo cardiovascular y la incidencia o no de insuficiencia cardiaca. Se llevaron a cabo exámenes de laboratorio, electrocardiograma y ecocardiografía Doppler tisular.
ResultadosEl resultado compuesto por muerte por cualquier causa u hospitalización por enfermedad cardiovascular se observó en el 11,4% de los individuos (53,1% mujeres). El BNP mantuvo la significación estadística incluso después de realizar un análisis de regresión de Cox múltiple para minimizar los efectos de las variables de confusión. El área bajo la curva ROC registrada para el BNP para identificar el resultado compuesto fue de 0,75 (IC95%, 0,672-0,817; p<0,0001). El valor de corte de BNP de 25pg/ml fue el resultado compuesto mejor correlacionado (sensibilidad: 72%; especificidad: 71%; precisión: 91%; valor predictivo negativo: 95%; razón de verosimilitud positiva: 24%).
ConclusionesEl estudio ha demostrado que el BNP es un predictor de muerte en pacientes de atención primaria con o sin insuficiencia cardiaca.
Primary care is the gateway to healthcare systems. It has direct impact on society's well-being and accounts for determining solutions to health issues, such as preventing and controlling chronic nontransmissible diseases. Chronic nontransmissible diseases have high incidence and mortality rate, and they often affect low-income populations with lower schooling level.1,2
Recent advancements in the diagnostic approach adopted in cardiology practice comprise the use of natriuretic peptides, such as B-type natriuretic peptide (BNP) and the N-terminal fraction of B-type natriuretic peptide, since these biomarkers are remarkably useful to monitor cardiovascular diseases, mainly in heart failure (HF) cases. HF was herein defined as progressive condition that starts with the incidence of cardiovascular risk factors; then, it evolves to asymptomatic structural and functional cardiac changes, to the onset of signs and symptoms, to disability and, finally, to death. Plasma natriuretic peptides concentrations have prognostic value for mortality and morbidity in patients with HF. BNP is also accurate in screening patients with left ventricular dysfunction and overall heart diseases. However, BNP prognostic cut-off values in primary care patients are yet to be established.3–5
BNP measurement is an easy, fast, and accurate technique, consolidated in primary care-related clinical practice diagnose HF. However, there is no consensus about the ideal cut-off point for BNP at this healthcare level. The European Society of Cardiology guideline has suggested the adoption of cut-off point for BNP of 35pg/mL, whereas the Canadian guideline establishes cut-off point of 50pg/mL, and the Brazilian guideline on HF establishes cut-off point >35–50pg/mL for outpatients.6–8
The aim of the current study was to assess the risk of cardiovascular events in primary care patients in the age group 45 years or older by measuring BNP regardless of the incidence of HF.
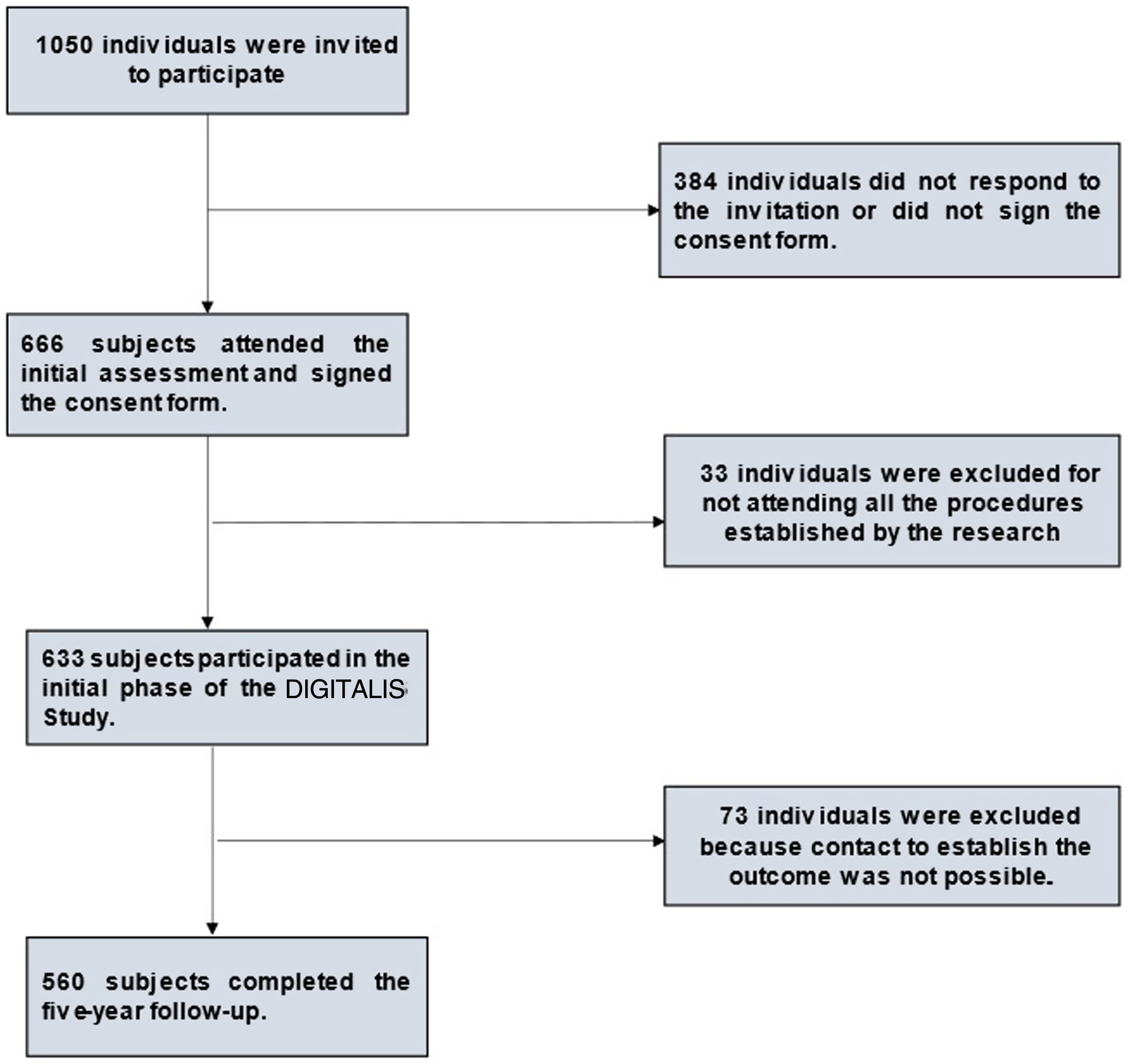
MethodsObservational study conducted with 560 individuals, 355 of them were women (63.4%), at mean age of 59.6±10.4 years, who were enrolled in the primary care program. It corresponds the phase II follow-up of the DIGITALIS study. Participants were followed-up for 4.5 years (maximum 4.9 years). Fig. 1 shows the flow chart of study design.
The DIGITALIS study took place in 2 different phases. Phase I comprised data collection carried out from July 2011 to December 2012. Male and female individuals, in the age group 45–99 years, who were enrolled in the family medical program, of a medium-sized city in Brazil were included in the study at this phase.
Exclusion criteria set for phase I comprised: pregnancy and conditions that prevented patients’ attendance at the health unit for examination purposes.
All individuals selected in phase I underwent assessment, which was carried out in a single day based on the following procedures: medical consultation and clinical examination, nursing consultation, anthropometric assessment, food frequency questionnaire application, DIGITALIS questionnaire completion and laboratory tests.
BNP was measured in all individuals who participated in phase I, based on the triage BNP Test (BioSite, Boston, USA), which is a fast fluorescence immunoassay test, results were expressed as pg/mL.
All participants underwent simultaneous 12-lead resting electrocardiogram conducted in GE MAC 800 device (GE MAC 800 1, 2, 4, 12 SL V239-USA), and 2-dimensional transthoracic echocardiogram with tissue Doppler imaging conducted in 2 different devices (Cypress 20 Acuson/Siemens-USA and Esaote AU-3 Partner-Italy). Recommendations from the American Society of Echocardiography, and from the European Association of Echocardiography for cardiac chamber quantification were here in applied.9
Phase II of the DIGITALIS study was carried out from January 2016 to September 2017 in order to establish a primary composite outcome, which was defined as all-cause death or hospitalization due to cardiovascular disease.
DIGITALIS study protocols were approved by the Research Ethics Committee of the Medical School/Hospital Universitário Antonio Pedro, CAAE: 0077.0.258.000-10 and CAAE: 49637115.7.0000.5243 and informed written consent was provided by all participating patients.
Statistical analysis was performed in the SPSS 21.0 software (SPSS Inc., USA). Continuous variables with normal distribution were expressed as means±standard deviation, whereas those with nonnormal distribution were expressed as median and interquartile range. Categorical variables were represented as absolute and relative frequency. Student t test was used to compare numerical data about continuous variables with normal distribution, whereas Mann–Whitney test was applied to continuous variables without normal distribution. Chi-square test was used to compare categorical data, it was followed by Yates’ correction and Fisher's exact tests, whenever necessary. Raw Hazard ratios and their 95% confidence intervals (95%CI) were estimated based on Cox regression. Variables presenting significance level of 10% (P<.1) in the simple regression analysis were selected to investigate the ones independently associated with changes in the outcome. Variables selected in the simple models were included in the multiple models in the second phase, those showing P<.05 were retained, and then presented the adjusted hazard ratios and their 95%CI.
Kaplan–Meier survival curves were fitted by applying the log-rank test for differences between categories.
Sensitivity, specificity, accuracy, positive predictive value, negative predictive value (NPV), positive likelihood ratio, negative likelihood ratio was calculated, as well as the ROC (receiver operating characteristic) curve was fitted for different BNP levels. The area under the curve was presented at 95%CI.
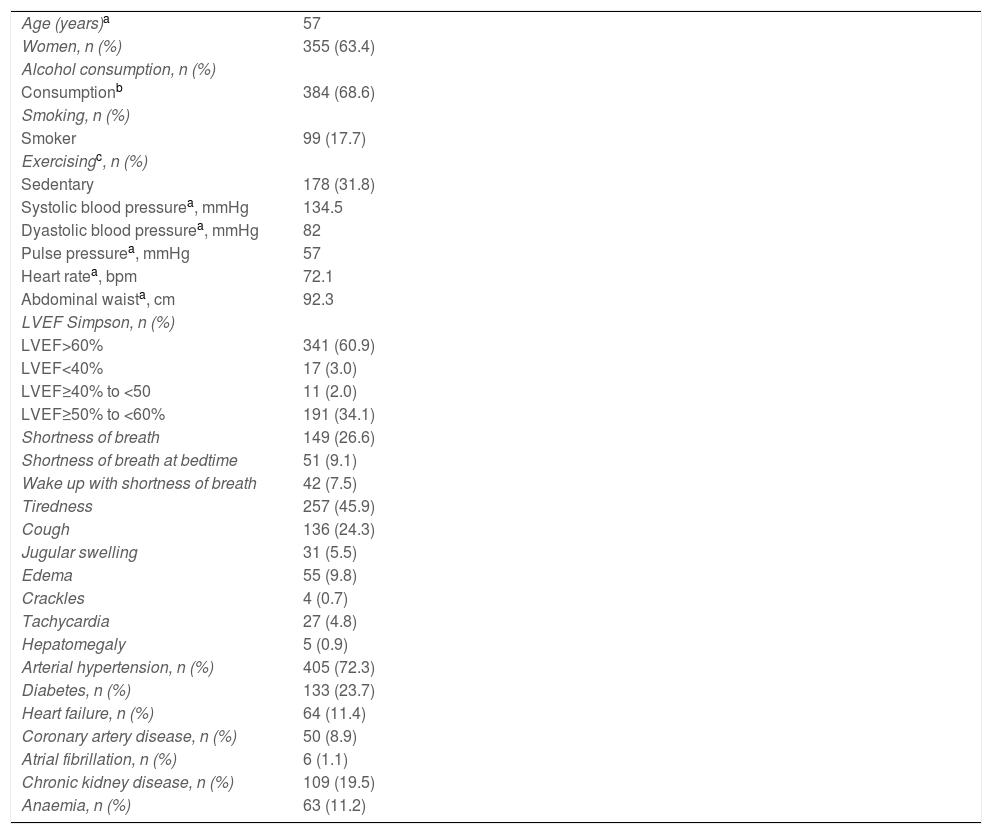
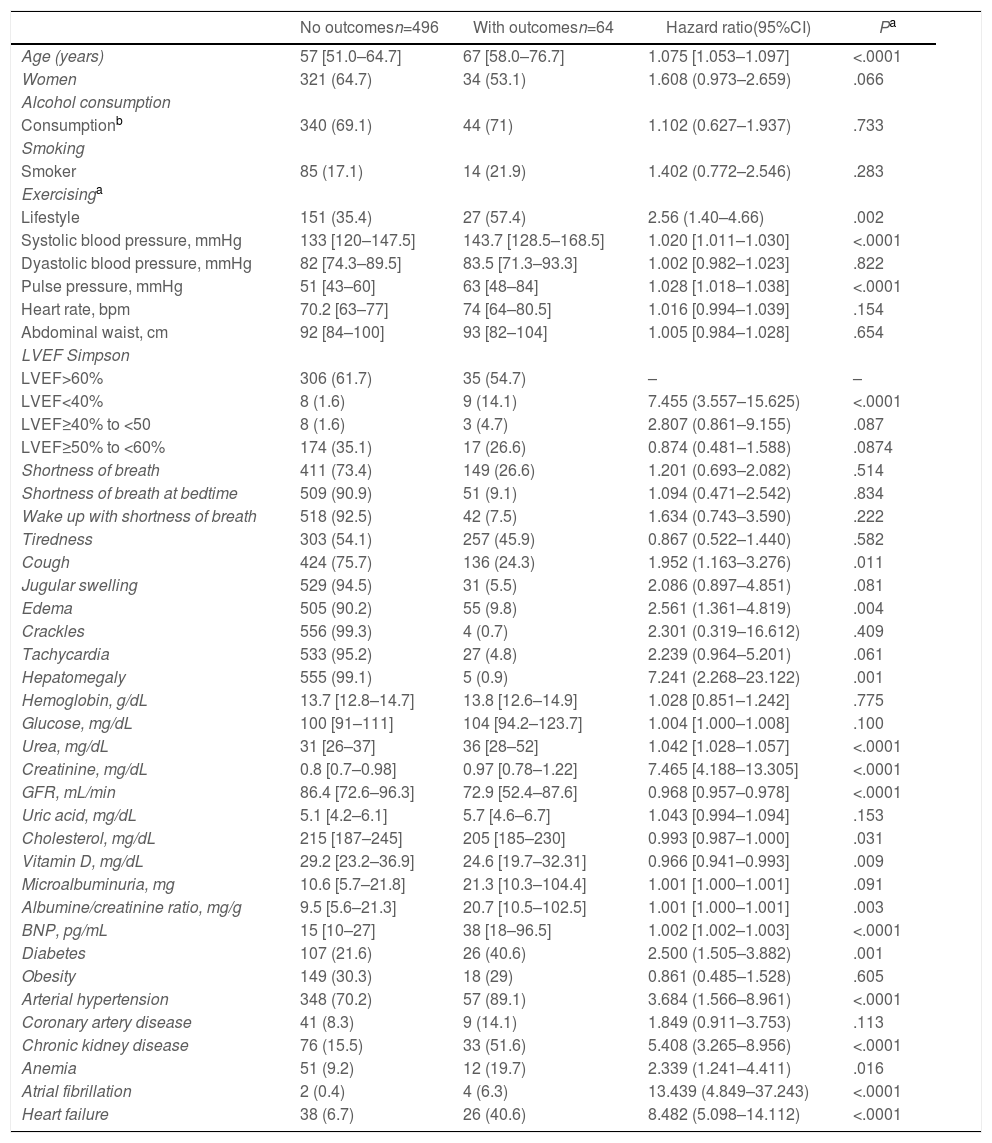
ResultsDemographic features, baseline, clinical data, comorbidities and/or risk factors of the investigated patients are shown in Table 1.
Demographic features, baseline, clinical data, comorbidities and/or risk factors of selected individuals.
| Age (years)a | 57 |
| Women, n (%) | 355 (63.4) |
| Alcohol consumption, n (%) | |
| Consumptionb | 384 (68.6) |
| Smoking, n (%) | |
| Smoker | 99 (17.7) |
| Exercisingc, n (%) | |
| Sedentary | 178 (31.8) |
| Systolic blood pressurea, mmHg | 134.5 |
| Dyastolic blood pressurea, mmHg | 82 |
| Pulse pressurea, mmHg | 57 |
| Heart ratea, bpm | 72.1 |
| Abdominal waista, cm | 92.3 |
| LVEF Simpson, n (%) | |
| LVEF>60% | 341 (60.9) |
| LVEF<40% | 17 (3.0) |
| LVEF≥40% to <50 | 11 (2.0) |
| LVEF≥50% to <60% | 191 (34.1) |
| Shortness of breath | 149 (26.6) |
| Shortness of breath at bedtime | 51 (9.1) |
| Wake up with shortness of breath | 42 (7.5) |
| Tiredness | 257 (45.9) |
| Cough | 136 (24.3) |
| Jugular swelling | 31 (5.5) |
| Edema | 55 (9.8) |
| Crackles | 4 (0.7) |
| Tachycardia | 27 (4.8) |
| Hepatomegaly | 5 (0.9) |
| Arterial hypertension, n (%) | 405 (72.3) |
| Diabetes, n (%) | 133 (23.7) |
| Heart failure, n (%) | 64 (11.4) |
| Coronary artery disease, n (%) | 50 (8.9) |
| Atrial fibrillation, n (%) | 6 (1.1) |
| Chronic kidney disease, n (%) | 109 (19.5) |
| Anaemia, n (%) | 63 (11.2) |
LVEF, left ventricular ejection fraction.
Older, sedentary individuals with left ventricular ejection fraction <40% have shown higher prevalence of composite outcomes (Table 2).
Demographic features, baseline, clinical, laboratory data, comorbidities and/or risk factors of selected individuals.
| No outcomesn=496 | With outcomesn=64 | Hazard ratio(95%CI) | Pa | |
|---|---|---|---|---|
| Age (years) | 57 [51.0–64.7] | 67 [58.0–76.7] | 1.075 [1.053–1.097] | <.0001 |
| Women | 321 (64.7) | 34 (53.1) | 1.608 (0.973–2.659) | .066 |
| Alcohol consumption | ||||
| Consumptionb | 340 (69.1) | 44 (71) | 1.102 (0.627–1.937) | .733 |
| Smoking | ||||
| Smoker | 85 (17.1) | 14 (21.9) | 1.402 (0.772–2.546) | .283 |
| Exercisinga | ||||
| Lifestyle | 151 (35.4) | 27 (57.4) | 2.56 (1.40–4.66) | .002 |
| Systolic blood pressure, mmHg | 133 [120–147.5] | 143.7 [128.5–168.5] | 1.020 [1.011–1.030] | <.0001 |
| Dyastolic blood pressure, mmHg | 82 [74.3–89.5] | 83.5 [71.3–93.3] | 1.002 [0.982–1.023] | .822 |
| Pulse pressure, mmHg | 51 [43–60] | 63 [48–84] | 1.028 [1.018–1.038] | <.0001 |
| Heart rate, bpm | 70.2 [63–77] | 74 [64–80.5] | 1.016 [0.994–1.039] | .154 |
| Abdominal waist, cm | 92 [84–100] | 93 [82–104] | 1.005 [0.984–1.028] | .654 |
| LVEF Simpson | ||||
| LVEF>60% | 306 (61.7) | 35 (54.7) | – | – |
| LVEF<40% | 8 (1.6) | 9 (14.1) | 7.455 (3.557–15.625) | <.0001 |
| LVEF≥40% to <50 | 8 (1.6) | 3 (4.7) | 2.807 (0.861–9.155) | .087 |
| LVEF≥50% to <60% | 174 (35.1) | 17 (26.6) | 0.874 (0.481–1.588) | .0874 |
| Shortness of breath | 411 (73.4) | 149 (26.6) | 1.201 (0.693–2.082) | .514 |
| Shortness of breath at bedtime | 509 (90.9) | 51 (9.1) | 1.094 (0.471–2.542) | .834 |
| Wake up with shortness of breath | 518 (92.5) | 42 (7.5) | 1.634 (0.743–3.590) | .222 |
| Tiredness | 303 (54.1) | 257 (45.9) | 0.867 (0.522–1.440) | .582 |
| Cough | 424 (75.7) | 136 (24.3) | 1.952 (1.163–3.276) | .011 |
| Jugular swelling | 529 (94.5) | 31 (5.5) | 2.086 (0.897–4.851) | .081 |
| Edema | 505 (90.2) | 55 (9.8) | 2.561 (1.361–4.819) | .004 |
| Crackles | 556 (99.3) | 4 (0.7) | 2.301 (0.319–16.612) | .409 |
| Tachycardia | 533 (95.2) | 27 (4.8) | 2.239 (0.964–5.201) | .061 |
| Hepatomegaly | 555 (99.1) | 5 (0.9) | 7.241 (2.268–23.122) | .001 |
| Hemoglobin, g/dL | 13.7 [12.8–14.7] | 13.8 [12.6–14.9] | 1.028 [0.851–1.242] | .775 |
| Glucose, mg/dL | 100 [91–111] | 104 [94.2–123.7] | 1.004 [1.000–1.008] | .100 |
| Urea, mg/dL | 31 [26–37] | 36 [28–52] | 1.042 [1.028–1.057] | <.0001 |
| Creatinine, mg/dL | 0.8 [0.7–0.98] | 0.97 [0.78–1.22] | 7.465 [4.188–13.305] | <.0001 |
| GFR, mL/min | 86.4 [72.6–96.3] | 72.9 [52.4–87.6] | 0.968 [0.957–0.978] | <.0001 |
| Uric acid, mg/dL | 5.1 [4.2–6.1] | 5.7 [4.6–6.7] | 1.043 [0.994–1.094] | .153 |
| Cholesterol, mg/dL | 215 [187–245] | 205 [185–230] | 0.993 [0.987–1.000] | .031 |
| Vitamin D, mg/dL | 29.2 [23.2–36.9] | 24.6 [19.7–32.31] | 0.966 [0.941–0.993] | .009 |
| Microalbuminuria, mg | 10.6 [5.7–21.8] | 21.3 [10.3–104.4] | 1.001 [1.000–1.001] | .091 |
| Albumine/creatinine ratio, mg/g | 9.5 [5.6–21.3] | 20.7 [10.5–102.5] | 1.001 [1.000–1.001] | .003 |
| BNP, pg/mL | 15 [10–27] | 38 [18–96.5] | 1.002 [1.002–1.003] | <.0001 |
| Diabetes | 107 (21.6) | 26 (40.6) | 2.500 (1.505–3.882) | .001 |
| Obesity | 149 (30.3) | 18 (29) | 0.861 (0.485–1.528) | .605 |
| Arterial hypertension | 348 (70.2) | 57 (89.1) | 3.684 (1.566–8.961) | <.0001 |
| Coronary artery disease | 41 (8.3) | 9 (14.1) | 1.849 (0.911–3.753) | .113 |
| Chronic kidney disease | 76 (15.5) | 33 (51.6) | 5.408 (3.265–8.956) | <.0001 |
| Anemia | 51 (9.2) | 12 (19.7) | 2.339 (1.241–4.411) | .016 |
| Atrial fibrillation | 2 (0.4) | 4 (6.3) | 13.439 (4.849–37.243) | <.0001 |
| Heart failure | 38 (6.7) | 26 (40.6) | 8.482 (5.098–14.112) | <.0001 |
95%CI, 95% confidence interval; BNP, B type natriuretic peptide; GFR, glomerular filtration rate calculated through the CKD-EPI formula; LVEF, left ventricular ejection fraction.
Cough, edema, and hepatomegaly were the signs and symptoms associated with composite outcome (Table 2).
Urea, creatinine, albumin/creatinine ratio and BNP were associated with composite outcomes. Cholesterol, vitamin D and glomerular filtration rate were statistically significant as protective factors in the composite outcome group (Table 2).
Diabetes mellitus, systemic arterial hypertension, chronic kidney disease (CKD), anemia, atrial fibrillation and HF were the comorbidities correlated to the incidence of composite outcome (Table 2).
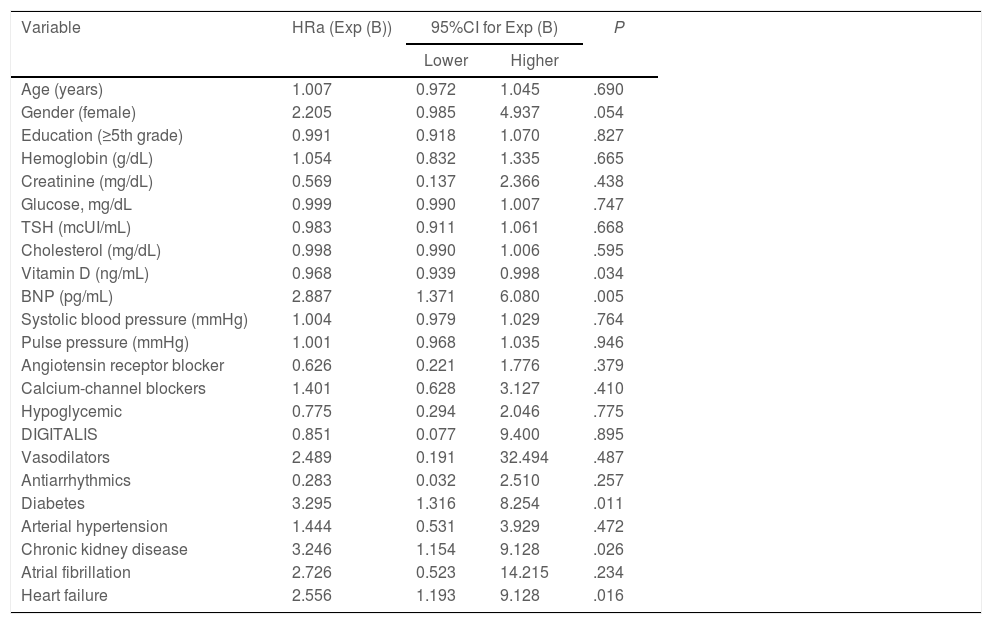
Multiple Cox regression analysis was used to evaluate the effect of independent variables on composite outcome (Table 3).
Multiple Cox regression analysis applied to composite outcome.
| Variable | HRa (Exp (B)) | 95%CI for Exp (B) | P | |
|---|---|---|---|---|
| Lower | Higher | |||
| Age (years) | 1.007 | 0.972 | 1.045 | .690 |
| Gender (female) | 2.205 | 0.985 | 4.937 | .054 |
| Education (≥5th grade) | 0.991 | 0.918 | 1.070 | .827 |
| Hemoglobin (g/dL) | 1.054 | 0.832 | 1.335 | .665 |
| Creatinine (mg/dL) | 0.569 | 0.137 | 2.366 | .438 |
| Glucose, mg/dL | 0.999 | 0.990 | 1.007 | .747 |
| TSH (mcUI/mL) | 0.983 | 0.911 | 1.061 | .668 |
| Cholesterol (mg/dL) | 0.998 | 0.990 | 1.006 | .595 |
| Vitamin D (ng/mL) | 0.968 | 0.939 | 0.998 | .034 |
| BNP (pg/mL) | 2.887 | 1.371 | 6.080 | .005 |
| Systolic blood pressure (mmHg) | 1.004 | 0.979 | 1.029 | .764 |
| Pulse pressure (mmHg) | 1.001 | 0.968 | 1.035 | .946 |
| Angiotensin receptor blocker | 0.626 | 0.221 | 1.776 | .379 |
| Calcium-channel blockers | 1.401 | 0.628 | 3.127 | .410 |
| Hypoglycemic | 0.775 | 0.294 | 2.046 | .775 |
| DIGITALIS | 0.851 | 0.077 | 9.400 | .895 |
| Vasodilators | 2.489 | 0.191 | 32.494 | .487 |
| Antiarrhythmics | 0.283 | 0.032 | 2.510 | .257 |
| Diabetes | 3.295 | 1.316 | 8.254 | .011 |
| Arterial hypertension | 1.444 | 0.531 | 3.929 | .472 |
| Chronic kidney disease | 3.246 | 1.154 | 9.128 | .026 |
| Atrial fibrillation | 2.726 | 0.523 | 14.215 | .234 |
| Heart failure | 2.556 | 1.193 | 9.128 | .016 |
95%CI, 95% confidence interval; BNP, B-type natriuretic peptide; HRa, adjusted hazard ratio; TSH, thyroid stimulating hormone.
BNP has maintained statistical significance (P=.005) in composite outcome, after multiple Cox regression application.
Multiple Cox regression has confirmed that risk factors for cardiovascular disease, such as diabetes mellitus and CKD are prognostic markers of cardiac injury rather than functional markers of cardiovascular health, such as BNP.
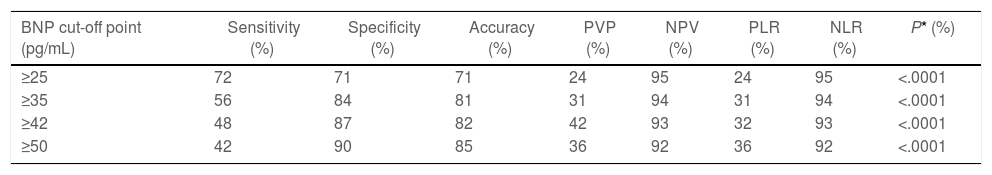
The present study evaluated the following BNP cut-off points (25, 35, 42, and 50pg/mL) (Table 4). Survival curve was statistically significant (P<.0001) in all cut-off points analyzed in the current study. There was statistically significant difference in survival rates between groups with and without composite outcomes.
Sensitivity, specificity, accuracy, PPV, NPV and positive and negative likelihood ratios recorded for different BNP cut-off points and composite outcome.
| BNP cut-off point (pg/mL) | Sensitivity (%) | Specificity (%) | Accuracy (%) | PVP (%) | NPV (%) | PLR (%) | NLR (%) | P* (%) |
|---|---|---|---|---|---|---|---|---|
| ≥25 | 72 | 71 | 71 | 24 | 95 | 24 | 95 | <.0001 |
| ≥35 | 56 | 84 | 81 | 31 | 94 | 31 | 94 | <.0001 |
| ≥42 | 48 | 87 | 82 | 42 | 93 | 32 | 93 | <.0001 |
| ≥50 | 42 | 90 | 85 | 36 | 92 | 36 | 92 | <.0001 |
BNP, B-type natriuretic peptide; NPV, negative predictive value; NLR, negative likelihood rate; PLR, positive likelihood rate; PPV, positive predictive value.
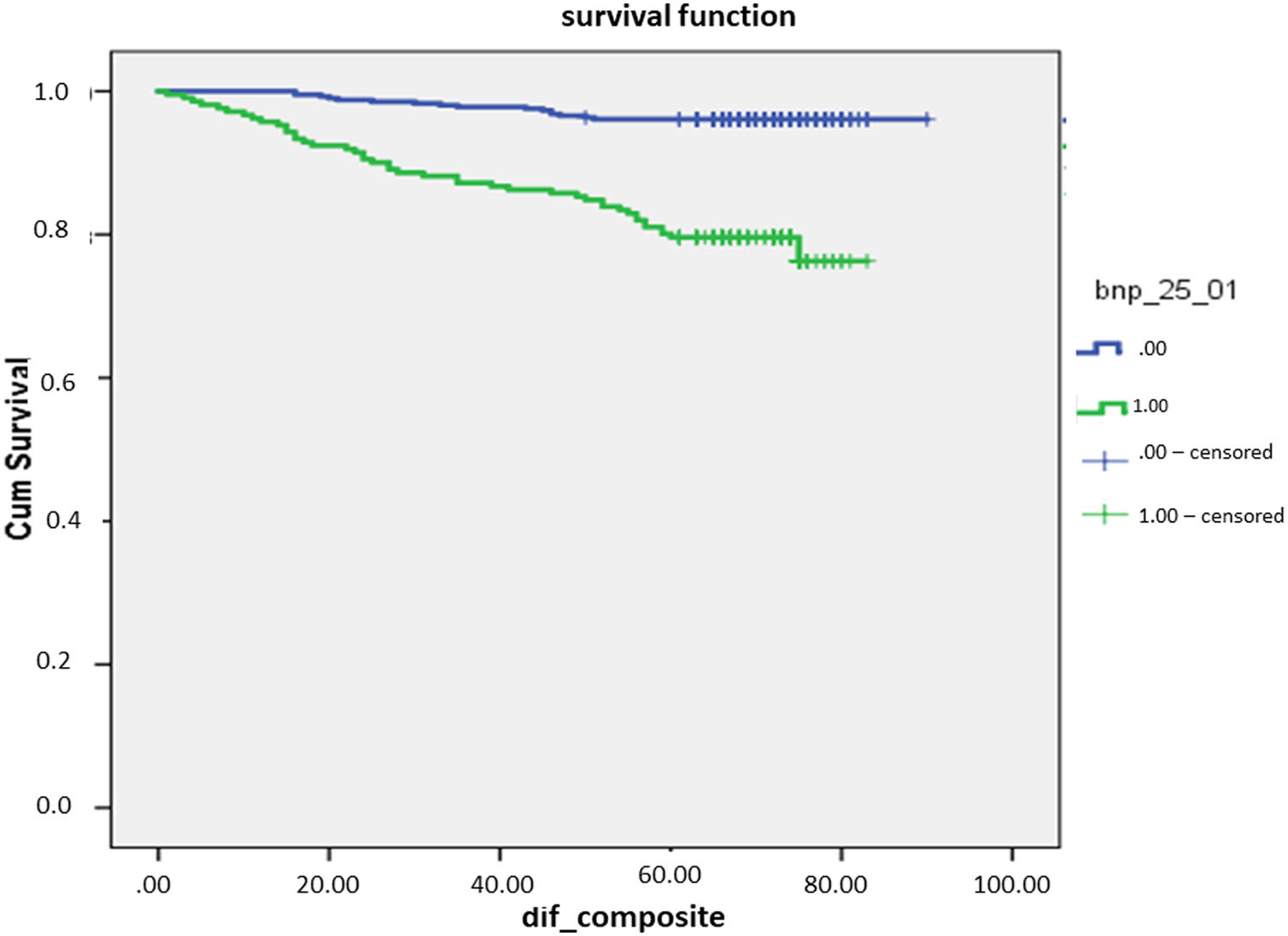
Fig. 2 shows the Kaplan–Meier plot for BNP cut-off point of 25pg/mL, which presented 72% sensitivity, 71% specificity, positive predictive value 24%, NPV 95% and positive likelihood ratio 24% to identify composite outcome (all-cause death or hospitalization due to cardiovascular disease). It was considered the ideal BNP cut-off point in the current study.
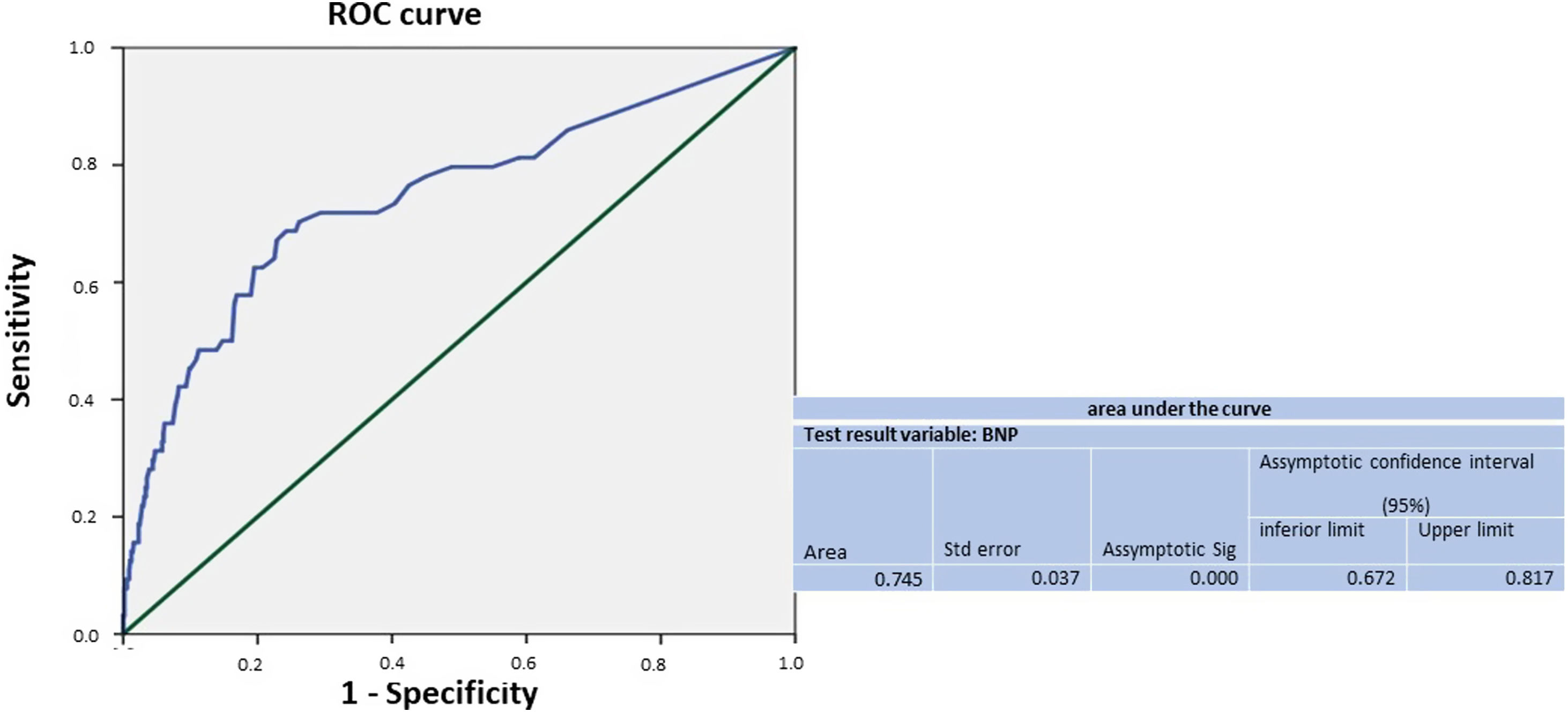
The area under the ROC curve observed for BNP to identify composite outcome was 0.75 (95%CI, 0.672–0.817; P<.001) (Fig. 3).
DiscussionThe main result in the study lied on the association between BNP measurement and the incidence of composite outcomes, such as all-cause death or hospitalization due to cardiovascular disease in primary care patients with and without HF.
Based on the here in presented data BNP cut-off point of 25pg/mL showed 72% sensitivity, 71% specificity, 95% NPV and area under the ROC curve of 0.75 (95%CI, 0.672–0.817; P<.001) to identify composite outcome in primary care patients with or without HF. The high NPV herein recorded for BNP, at cut-off point of 25pg/mL, enabled measuring this peptide to work as screening test to emphasize the incidence of composite outcome.
BNP level was statistically significant to detect composite outcome, regardless of HF incidence in primary care patients moreover plasma BNP levels were higher in the group with composite outcomes than in the one without them.
Patients assisted in primary care units were older and heterogeneous, as well as often oligosymptomatic in comparison to those followed up in specialized units. They frequently presented cardiovascular diseases symptoms, such as dyspnea, which is also a condition observed in other diseases.10 Thus, establishing diagnosis and/or prognosis only based on clinical assessment can be quite challenging.
Primary care services have limited availability of complementary exams, such as echocardiography due to lack of qualified professionals and specialized services.10 Thus natriuretic peptide measurement is an easy, fast, accessible, accurate, and low-cost method, capable of helping the early identification of unfavorable prognoses in patients with and without HF. This natriuretic peptide is the one that best expresses patients’ heart condition; moreover, it is a functional marker of cardiovascular health, therefore, it has strong clinical applicability.
Data analyzed in the present study have shown that BNP was a good prognostic marker in patients with CKD, diabetes mellitus and systemic arterial hypertension, regardless of the incidence of HF.
The current study has analyzed the survival curve for different BNP cut-off points, 25pg/mL, 35pg/mL, 42pg/mL, and 50pg/mL, and found difference in survival rates between individuals with and without composite outcomes at all cut-off points.
The likelihood of finding composite outcomes has increased by 0.5%.
The current data have shown that individuals with composite outcomes who presented the lowest BNP levels (25 and 35pg/mL) recorded survival rate lower than that of individuals presenting the highest BNP levels (42 and 50pg/mL).
Bassan et al. have justified the use of BNP measurement because the left ventricular systolic and/or diastolic dysfunction precedes myocardial cell death.11
Framingham study conducted in the community was a prospective research that included 3346 patients with BNP levels >20pg/mL, without HF, for 5.2 years; and results have shown that BNP was a good predictor of cardiovascular events. This outcome was similar to the one observed in the present study, which showed incidence of composite outcome at BNP cut-off point of 25pg/mL, however, there was difference in research type and population sample between the aforementioned studies.12
Study conducted by Hejl et al. included 61 665 Danish primary care patients (55.6% were women), at mean age 64.9 years, who were followed-up for 4.36 years, reported lower survival rates in those with plasma BNP concentration >35pg/mL. This outcome was different from the one reported in the current study, which recorded lower survival rate for patients with BNP equal to 25pg/mL. Although there is similarity in the follow-up time adopted in both studies, they used different sample sizes.13
The study conducted by Alehagen et al. in a rural population comprised 474 primary care patients, at mean age of 73 years, who presented signs and symptoms of HF and who were followed-up for 5.5 years. Patient survival recorded lower rates at the highest BNP quartiles. Unlike the current study, the aforementioned authors used an interquartile range. Another point of divergence between both studies lies on the fact that the population investigated in the current study was 100% urban.14
Tsuchida et al. assessed 3123 patients, at mean age 59.3±15.3 years, who were treated at a general practice clinic in Japan and followed-up for 5.5 years. The following BNP cut-off points were analyzed: 20pg/mL, 40pg/mL, 100pg/mL, 200pg/mL, and 500pg/mL. BNP level ≥100pg/mL was correlated to composite outcome and to all-cause mortality, regardless of heart disease incidence. This finding was similar to the one reported in the current study; however, the BNP cut-off point used in our study's assay in order to detect composite outcome was 25pg/mL. In addition, patients’ mean age was older and follow-up time was longer in the Japanese study.4
STOP-HF study performed with primary care patients has shown that those whose BNP dosage was >50pg/mL died due to cardiovascular outcomes and were more often hospitalized due to acute decompensation.15
The cut-off point of 25pg/mL recorded in the current study is specific to identify composite outcomes, such as all-cause death or hospitalization due to cardiac disease. In addition, it has negative predictive value of 95%, which turns BNP into a screening test to identify the incidence of composite outcomes in primary care patients with and without HF.
Noncommunicable diseases are a chronic health issue due their high incidence and prevalence, as well as because they impose significant expenses on healthcare systems. Although simple complementary tests, such as blood glucose, urea, uric acid, microalbuminuria, albumin/creatinine ratio, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol are widely available variables, they are not predictors of composite outcome (all-cause death or hospitalization due to cardiovascular disease).
The BNP is a very sensitive biomarker released by the heart ventricles in the face of minimum aggressions. In decompensated HF, congestion is the main stimulus for releasing natriuretic peptides. However, in patients without overt HF, BNP may be released in small amounts by early functional damages caused by risk factors such as diabetes, hypertension, and ischemia. Of note, the values of BNP may increase even before systolic dysfunction is detected by echocardiography. For these reasons, BNP is an early marker of prognosis.
The early identification of individuals at risk of developing composite outcomes regardless of the HF incidence at the gateway to the healthcare system, based on easy, quick, and accessible test, such as BNP dosage helps reducing health related expenses, improving patients’ quality of life and their individual and collective well-being, as well as rationalizing the use of high-cost technologies.
Although BNP is useful as prognostic tool in primary care, one should not forget that physicians should monitor the incidence of cardiovascular symptoms and make significant efforts to control cardiovascular risk factors.
LimitationsThe current study should be interpreted considering its limitations, since it is a retrospective study. Although BNP is a good composite outcome marker, it was not possible to identify the structural cardiac changes, therefore, future studies should be carried out to validate these findings.
ConclusionsBNP is a strong all-cause mortality or cardiovascular hospitalization predictor in the primary healthcare setting, regardless of HF incidence at baseline; thus, 25pg/mL was set as the best cut-off point for such a purpose. BNP incorporation to primary healthcare may be useful to identify patients at the highest risk of having cardiovascular events in the community, who could benefit from preventive strategies.
- •
Prevention strategies for cardiovascular diseases are necessary in primary care.
- •
Chronic non-communicable diseases cause large expenses to the health system and increase the risk of developing heart failure.
- •
The use of a low-cost complementary test such as BNP can help in the early identification of patients at higher cardiovascular risk.
Early identification of individuals at risk of developing outcomes regardless of the presence of HF, at the gateway to the health system, before admission to advanced levels of care, through an easy, quick and accessible test such as BNP measurement, reduces health expenditures, promotes improvement in quality of life and individual and collective well-being, and rationalizes the use of high-cost technologies.
This study was partially funded by the following institutions: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes, Brazil) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (Faperj, Brazil).
Authors’ contributionsA.P. Arriaga Carvalho Salles: contributions to the conception or design of the work; interpretation of data for the work. H. Villacorta: final approval of the version to be published. W. de Andrade Martins: final approval of the version to be published. E. Tinoco Mesquita: contributions to the conception or design of the work. A. Renato Leite: contributions the acquisition, of data for the work. D.M. da Silva Correa: contributions the acquisition, of data for the work. M.L. Garcia Rosa: contributions to the acquisition, analysis, or interpretation of data for the work. M. Luiz Ribeiro: contributions to the interpretation of data for the work. A.J. Lagoeiro Jorge: contributions to the conception and design of the work; interpretation of data for the work.
Conflicts of interestNone.