Cardiovascular disease (CVD) continues to be a threat to global health as the leading cause of mortality worldwide.1 Diabetes is a well stablished risk factor for CVD which is the most common complication in diabetes.2 While metformin continues to be the first-line therapy for type 2 diabetes, new anti-diabetic medications have become approved. Diabetes is diagnosed according to fixed glucose and hemoglobin A1c (HbA1c) levels. After acknowledging that subjects not fulfilling the diabetes diagnostic criteria but having glucose and/or HbA1c values close to the thresholds have also an increased risk for CVD and other complications, the concept of prediabetes began to evolve. Far more recently, it has been demonstrated that the associations between glycemia and vascular injury is a continuum, with subclinical atherosclerosis being directly correlated with glucose and HbA1c levels even in ranges considered normal.3 In fact, very recently it has been demonstrated that in apparently healthy individuals (i.e. not [pre]diabetics), metabolic syndrome traits are associated with reactive bone marrow activation and early subclinical atherosclerosis.4 Having this in background, it is now easier to understand why interventions targeting glucose metabolism can have benefits extending diabetic subjects.
The impact of antidiabetic agents on CVD outcomes has received lot of attention in the past. More than a decade ago, a systematic review found that the use of rosiglitazone (drug used to treat type 2 diabetes) was associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes.5 Despite the analysis had important limitations (as acknowledged by authors), this study raised a warning with regards to the cardiovascular safety of novel antidiabetic agents. In fact, when introducing novel antidiabetic agents, it was asked to perform a complete cardiovascular safety assessment. Surprisingly (at that moment), new antidiabetic agents, such as sodium-glucose cotransporter 2 inhibitors (SGLT2i) where not associated with increased incidence of cardiovascular outcomes but had the complete opposite effect on these. In fact, in the EMPA-REG OUTCOME trial, an unexpected large benefit (35% relative risk reduction) in hospitalization for heart failure (HF) was found in type 2 diabetic patients with high CVD risk randomized to the SGLT2i empagliflozin.6 Canagliflozin and dapagliflozin, 2 additional SGLT2is, were studied in the large cardiovascular outcome trials (CANVAS7 and DECLARE-TIMI 588), confirming the benefit of these class of drugs on HF hospitalization and CVD death. Importantly, these latter studies included people with type 2 diabetes mellitus with variable degrees of CVD risk. After these fantastic results, dedicated trials such as DAPA-HF9 and EMPEROR-reduced10 demonstrated the benefit of these agents in patients with preexisting HF and reduced ejection fraction, with or without diabetes. More recently, the benefits of SGLT2i in HF was extended to those with preserved ejection fraction (HFpEF), as shown in EMPEROR-preserved11 and DELIVER12 trials. Additionally, emerging data has proposed that dipeptidyl peptidase-4 inhibitors (DPP4i) and glucagon-like peptide 1 receptor agonists (GLP-1RA) may be also provide cardioprotective benefits. Whether all new antidiabetic agents provide the same benefit in terms of reduction in CVD events is the matter of research.
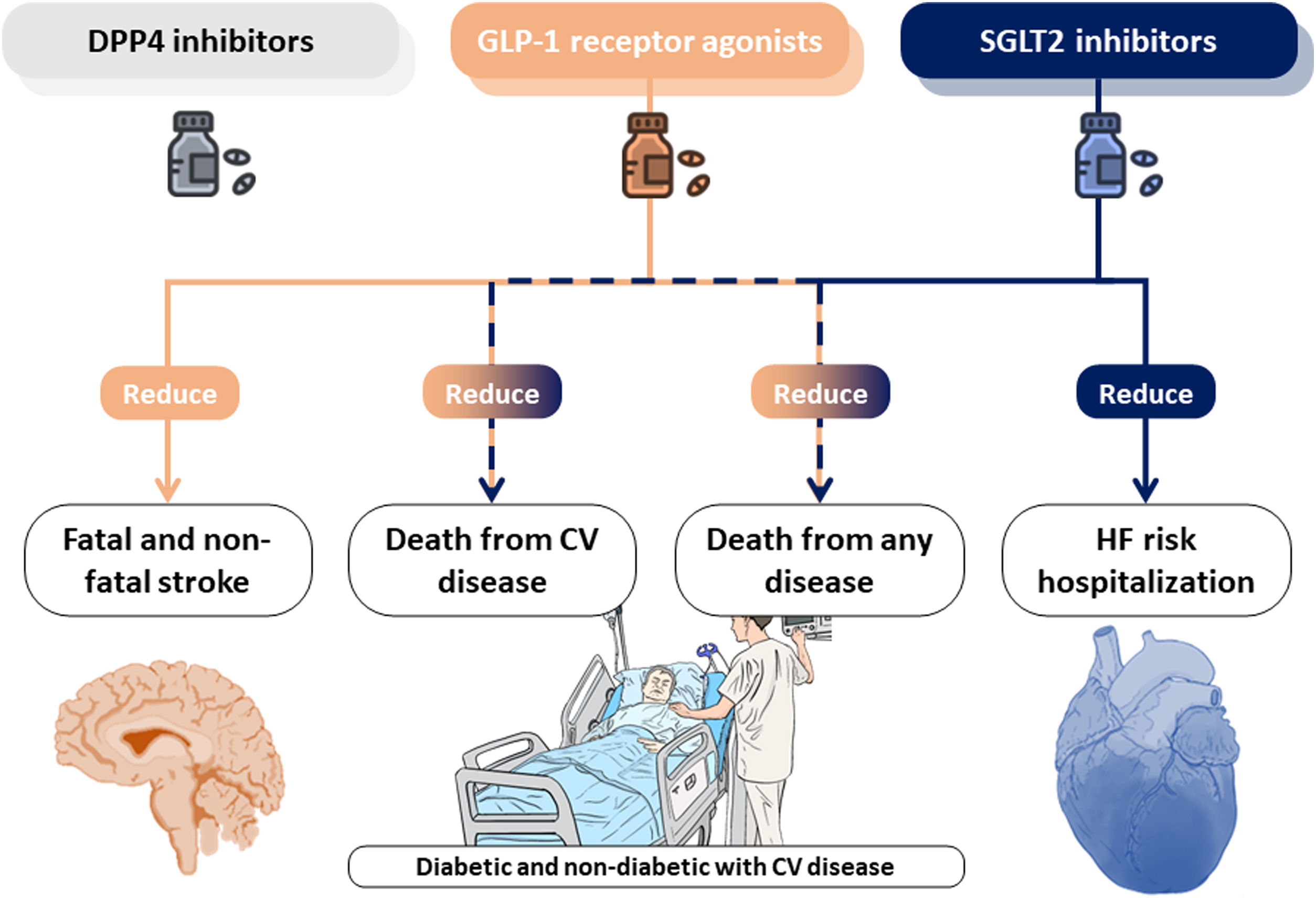
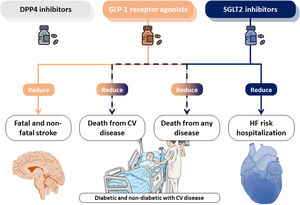
Kanie et al. conducted a standard and network meta-analysis of randomized control trials (20 studies including 124465 participants) to evaluate the effects of SGLT2i, DPP4i and GLP-1RA on cardiovascular outcomes in patients with established CVD.13 Their outcomes focused on cardiovascular mortality, fatal or non-fatal myocardial infarction, fatal and non-fatal stroke, all-cause mortality, hospitalization for HF and safety outcomes. DPP4i was not found to reduce the risk of any of the study outcomes, however, GLP-1RA and SGLT2i may represent potential treatment strategies for CVD. Data from both the standard and network meta-analysis suggests that GLP-1RA and SGLT2i are likely to reduce the risk of CVD mortality and all-cause mortality in people with established CVD. Regarding HF, only SGLT2i were able to reduce the risk of hospitalization for this cause. The lack of direct comparisons between each class of the agents limits the findings from this network meta-analysis.
While the CVD benefits of novel antidiabetic agents in subjects, mainly in terms of HF reduction, is well established, the mechanism responsible for this effect remains elusive. In the last years, several groups have been pursuing a mechanistic justification that could explain how these antidiabetic drugs are effective in treating CVD beyond diabetic patients. In the case of SGLT2i several canonical and non-canonical action pathways have been proposed.14 Control of glycemia, body weight or arterial pressure seem to be insufficient explanations on reducing cardiovascular events compared with other anti-diabetic drugs. Thus, the following possible mechanisms have emerged: a direct effect on cardiac cells that could reverse cardiac remodeling, improved myocardial energetics, mobilization of ketone bodies from the liver and its utilization by cardiomyocytes to increase the adenosine triphosphate production, an effect on ionic homeostasis and autophagy induction. Collectively, these mechanisms which optimize cardiac metabolism and substrate utilization (altered in both types of HF, with preserved or reduced ejection fraction) seem to be the more feasible and reproducible in patients and animal models.15–17 In the case of GLP-1RA there is less evidence focused on myocardium and CV model of disease.18 Regarding HFpEF, left ventricular EF remained unchanged and no differences in HF hospitalization were found in different clinical trials. All this make SGLT2i a more promising therapeutic tool in HF field and some retrospective observational studies have pointed its potential targeted-specific diseases causing cardiac dysfunction such as cardiotoxicity secondary to anthracycline chemotherapy.
It is necessary to develop both clinical and preclinical studies to explore the cardioprotective mechanism behind these promising antidiabetic drugs. Better understanding these underlying mechanisms can lead to the rapid repurposing of these already approved drugs. Patient should, certainly, directly benefit from the reverse-translational wheel beyond the real-data clinical research. A central illustration of this editorial is summarized in Fig. 1.
B. Ibáñez is supported by the European Commission (H2020-HEALTH grant N° 945118 and ERC-CoG grant N° 819775) and by the Spanish Ministry of Science and Innovation (MCN; ‘RETOS 2019’ grant N° PID2019-107332RB-I00), and by the Spanish Society of Cardiology. The CNIC is supported by the ISCIII, the Ministerio de Ciencia e Innovación, and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (CEX2020-001041-S).
Conflicts of interestNone.
This article is based on a Cochrane Review previously published in the Cochrane Database of Systematic Reviews 2021, Issue 10, Art. No.: CD013650, doi:10.1002/14651858.CD013650.pub2 (see https://www.cochranelibrary.com/ for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.