Remote monitoring (RM) is a new tool in heart failure (HF) patients with cardiac implantable electronic devices but its effect in clinical outcomes is still uncertain. In remote regions as islands HF management is even more challenging and RM may have a different impact. We aimed to assess its impact in clinical outcomes in an insular reality.
MethodsThis was a retrospective, non-randomized study conducted in our hospital, that is the reference center of a nine-island archipelago. Patients in the HF remote monitoring program were matched 1:1 with usual standard of care patients.
ResultsFrom 307 patients, 96 with RM (group 1 – G1) were matched 1:1 according to age ±2 years and gender with 96 usual standard of care (group 2 – G2) (mean age 69 years, 76% males, mean follow-up period 55 months). Primary endpoint was cardiovascular (CV) mortality and secondary endpoints all-cause mortality, HF hospitalizations at 12 months and at FUP. CV mortality was lower in G1 (hazard ratio [HR] 6.7; 95% confidence interval [95% CI] 1.46–30.87; P=.004), as well as all-cause mortality (HR, 5.7; 95% CI, 1.85–17.39; P<.001) and HF hospitalizations at 12 months (P=.02) with a tendency toward less hospitalizations during follow-up.
ConclusionsRM in the management of HF patients with cardiac implantable electronic devices in a remote geographic location, as a nine-island archipelago, was effective in reducing CV mortality, all-cause mortality and HF hospitalizations.
La monitorización remota (MR) es una nueva herramienta en pacientes con insuficiencia cardiaca (IC) con dispositivos cardiacos electrónicos implantables (DCEI), pero su efecto en los resultados clínicos aún es incierto. En regiones remotas, como las islas, la gestión de la IC es aún más desafiante y la MR puede tener un impacto diferente. Nuestro objetivo fue evaluar su impacto en los resultados clínicos en una realidad insular.
MétodosEstudio retrospectivo, no aleatorizado, realizado en nuestro hospital, que es el centro de referencia de un archipiélago de nueve islas. Los pacientes del programa de monitorización remota de IC se emparejaron 1:1 con los pacientes con el estándar de atención habitual.
ResultadosDe 307 pacientes, 96 en MR (grupo 1 [G1]) se emparejaron 1:1 según edad ±2 años y sexo, con 96 pacientes con estándar de atención habitual (grupo 2 [G2]) (edad media 69 años, 76% hombres, periodo de seguimiento medio de 55 meses). El objetivo primario fue la mortalidad cardiovascular (CV), y los objetivos secundarios, la mortalidad por todas las causas, las hospitalizaciones por insuficiencia cardiaca a los 12meses y en seguimiento. La mortalidad CV fue menor en G1 (hazard ratio [HR]=6,7; intervalo de confianza del 95% [IC 95%]: 1,46-30,87; p=0,004) así como la mortalidad por todas las causas (HR=5,7; IC 95%: 1,85-17,39; p<0,001) y hospitalizaciones por IC a los 12meses (p=0,02), con tendencia a menos hospitalizaciones durante el seguimiento.
ConclusionesLa MR en el abordaje de pacientes con IC con DCEI en una región geográfica remota como un archipiélago de nueve islas fue eficaz para reducir la mortalidad CV, la mortalidad por todas las causas y las hospitalizaciones por IC.
Despite advances in treatment and management of heart failure (HF), the incidence of hospitalizations and mortality related to HF remains high.1 Cardiac implanted electronic devices (CIEDs) as cardiac resynchronization therapy (CRT) and implanted cardioverter-defibrillators (ICD) have become an important part of treatment of HF with reduced ejection fraction.2
Remote monitoring (RM) is a new paradigm in HF patients with CIEDs and it encompasses the continuous acquisition of clinical data and device parameters from a CIED and the storage and transmission to a remote interface, where it is accessible to the health team. RM includes the detection of information related to the device function (device malfunction, battery status, lead integrity and pacing threshold), detection of arrhythmias and parameters related to patient physiology (continuous heart rate, heart rate variability and activity, respiratory rate and in some cases heart sounds, intrathoracic impedance, relative tidal volume, physical activity and apnea and hypopnea index). In most cases, decompensation of HF is caused by elevation in central venous and cardiac filling pressures with volume overload that often precedes clinical congestion.3 HF fluid retention algorithms that integrate intrathoracic impedance measurements have been developed and generate automatic alerts that are send to the clinician whenever fluid retention thresholds are reached.4–6 A transmission can be prescheduled, initiated by the patient or driven by pre-defined alert events.7
Data obtained from RM may help identify earlier signs of HF decompensations, allowing an attempted and earlier optimization of medical therapy, preventing adverse clinical outcomes. Several studies and meta-analysis comparing device remote monitoring with usual care did not reveal an unequivocal reduction in mortality or HF hospitalizations.8–14
RM is recommended to reduce in-office follow-ups in patients with pacemakers who have difficulty to attend in-office visits and should be considered to provide earlier detection of clinical problems.15 And RM in HF may be considered to reduce the risk of CV and HF hospitalizations and CV mortality.2
In remote geographic regions such as islands, lack of specialized medical facilities and long distances to tertiary centers are often a reality, making HF management even more challenging. RM in this setting could play a crucial role in identifying earlier signs of HF decompensation and enhancing optimization of care in these patients. There is very little data regarding the use of RM in an insular scenario. We propose with this study to assess the impact of this technology in the management of patients with chronic HF with reduced ejection fraction in a reality of a nine-island archipelago.
MethodsThis was a retrospective, non-randomized study conducted in our hospital, that is the reference center of a nine-island archipelago located across a large geographically area where transport between islands is only available by airplane. Chronic HF patients followed in our center were eligible if they fulfilled all the following criteria: over 18 years old; previously implanted ICD, CRT pacemaker (CRT-P) or cardiac resynchronization defibrillator (CRT-D) device for primary or secondary prevention implanted at least 30 days before the enrolment and left ventricular ejection fraction (LVEF) ≤40%. All patients with devices implanted between January 2005 and February 2022 were included. Patients with a follow-up of less than 30 days were excluded. Patients were assessed through clinical in-office visits according to international recommendations and, when applicable, through continuous monitoring data from CIEDs transmitted remotely to the care team during the last 12 months. Transmissions were prescheduled, initiated by the patient, or driven by pre-defined events.
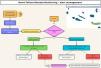
A structured HF management program was in use and whenever a RM alert was detected, the alert was initially assessed to exclude false alerts. After this a telephone assessment was conducted to assess the likelihood of a decompensation. If a decompensation was likely an in-office visit to the HF clinic in the main island or to the local health unit in a peripheral island to assess the patient and optimize treatment was completed and when necessary, the patients in the peripheral islands could be transferred by airplane to our center. The alert management is illustrated in Fig. 1. From the nine islands, two have hospital facilities with local cardiology and they receive patients from the closest islands. Six of the islands do not have hospital facilities and only have local general practitioner health care units. Treatment optimization was performed according to HF international recommendations.
Patients enrolled in the HF remote monitoring program were matched 1:1 with usual standard of care patients. The following parameters were included as propensity score matching criteria: age ±2 years and gender. The matching without replacement method was used. The best matched control was selected to maximize the accuracy of the analysis. Patients with missing information in any of these variables were excluded from the matching procedure.
Two groups were defined: standard of care plus RM (group 1 – G1), and usual standard of care (group 2 – G2). Standard of care was defined as usual standard of care with in-office visits according to international recommendations2 and optimized heart failure therapy. Patients with inactive RM were considered in the usual standard group. Two remote monitoring systems were used, LATITUDE NXT (Boston Scientific, United States) and CareLink (Medtronic, United States).
Clinical outcomes were retrospectively accessed since the implantation of the CIED until the last RM or in-office assessment, during the study period. Study data was obtained from the electronical medical records and from CIEDs interrogation. A subanalysis of patients with RM and active fluid retention algorithms was performed to assess the relation between the number and duration of fluid retention alerts and clinical outcomes.
The primary study endpoint was defined as CV mortality and secondary study endpoints were defined as all-cause mortality, unplanned HF hospitalizations at 12 months and during the follow-up period and improvement in New York Heart Association (NYHA) functional class.
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and interquartile ranges for variables with skewed distributions. Normal distribution was checked using Shapiro–Wilk test or skewness and kurtosis. All reported P values are two-tailed, with a P=.05 indicating statistical significance. Comparison of data was performed using the unpaired Student's t test for continuous variables and chi-square test for categorical variables. Cumulative survival curves for time-to-event analyses were constructed by the Kaplan–Meier method. Spearman's correlation coefficient was used to assess correlations. Analyses were performed with the use of SPSS software, v 27 (IBM, United States).
ResultsA total of 271 patients were included in the analysis. Seventy-nine were excluded from matching resulting in 192 patients included in the final analysis, with 96 in the RM group (G1) matched 1:1 to 96 in the usual standard of care group (G2), according to age (±2 years) and gender.
In G1 the remote monitoring system was LATITUDE NXT in 73 (76.0%) and CareLink in 23 (24.0%) patients. The mean age was 69 years, 76% of the patients were male, mean follow-up was 55 months and 87.0% of the patients were from the main island. Most patients were in New York Heart Association (NYHA) classification II (46.5%), mean N-terminal-pro hormone BNP (NT-proBNP) was 4503±6690pg/mL and mean LVEF was 34.5%. All baseline characteristics are summarized in Table 1.
Demographic and clinical characteristics of the patients at baseline and of group 1 (remote monitoring) and 2 (usual standard of care).
| Baseline characteristics | Population(n=192) | Group 1(n=96) | Group 2(n=96) | P |
|---|---|---|---|---|
| Follow-up (months) | 55.1±46.73 | 58.2±45.40 | 52.0±48.05 | ns |
| Age (years) | 69.1±10.20 | 68.9±10.40 | 69.2±10.22 | ns |
| Gender male | 146 (76.0) | 73 (76.0) | 73 (76.0) | ns |
| Main island | 167 (87.0) | 79 (82.3) | 88 (91.7) | ns |
| Peripheral island | 25 (13.0) | 17 (17.7) | 8 (8.3) | ns |
| CRT-D | 96 (50.0) | 51 (53.1) | 45 (46.9) | ns |
| ICD | 77 (40.1) | 40 (41.7) | 37 (38.5) | ns |
| CRT-P | 19 (9.9) | 5 (5.2) | 14 (14.6) | ns |
| HF etiology | ||||
| Ischemic | 58 (34.5) | 26 (27.1) | 32 (33.3) | ns |
| Non-ischemic | 86 (44.8) | 36 (37.5) | 50 (52.1) | ns |
| Familiar | 10 (6.0) | 3 (3.1) | 7 (7.3) | ns |
| Valvular | 3 (1.8) | 1 (1.0) | 2 (2.1) | ns |
| Atrial fibrillation | 71 (37.0) | 30 (31.3) | 41 (42.7) | ns |
| Coronary artery disease | 79 (41.1) | 33 (44.0) | 46 (47.9) | ns |
| Severe valvular disease | 7 (3.6) | 3 (3.1) | 3 (3.1) | ns |
| Peripheral artery disease | 14 (7.3) | 5 (5.2) | 9 (9.4) | ns |
| Hypertension | 115 (68.5) | 49 (51.0) | 55 (57.3) | ns |
| Dyslipidemia | 105 (54.7) | 44 (45.8) | 61 (63.5) | ns |
| Diabetes | 72 (37.5) | 34 (35.4) | 38 (39.9) | ns |
| Obesity | 59 (36.0) | 27 (28.1) | 43 (44.8) | ns |
| Chronic renal disease | 27 (14.1) | 10 (10.4) | 17 (17.7) | ns |
| History of smoking | 77 (40.1) | 34 (35.4) | 43 (44.8) | ns |
| Mean LVEF | 34.5±11.05 | 32.0±11.57 | 34.6±10.55 | ns |
| NYHA class at baseline | 2.1±0.79 | 2.2±0.79 | 2.1±0.83 | ns |
| NT-proBNP (pg/mL) | 4503±6690 | 2696±2654 | 5216±7645 | ns |
| Pharmacological treatment | ns | |||
| ACEi/ARB/ARNi | 183 (95.3) | 92 (95.8) | 91 (94.8) | ns |
| MRA | 144 (75.0) | 75 (78.1) | 69 (71.9) | ns |
| BB | 180 (93.8) | 91 (94.8) | 89 (92.7) | ns |
| SGLT2i/GLP-1 | 80 (41.7) | 37 (38.5) | 43 (44.8) | ns |
ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; ARNi: angiotensin receptor-neprilysin inhibitor; BB: beta-blockers; CRT-D: cardiac resynchronization defibrillator; CRT-P: cardiac resynchronization therapy pacemaker; GLP-1: glucagon-like peptide-1 agonists; ICD: transvenous implantable cardioverter-defibrillator; LVEF: left ventricle ejection fraction; MRA: mineralocorticoid receptor antagonists; NT-proBNP: N-terminal-pro hormone BNP; NYHA: New York Heart Association functional class; SGLT2i: sodium-glucose cotransporter 2 inhibitors.
Data are expressed as no. (%) or mean±standard deviation.
A summary of events is summarized in Table 2. Vital-status verification was completed for 100% of patients. Primary outcome defined as CV mortality occurred in 14 (8.8%) patients. All-cause mortality occurred in 23 (14.4%) patients. There were 32 unplanned HF hospitalization in the first 12 months, that occurred in 20 patients. Each patient had 0.2±0.60 unplanned HF hospitalizations in the first 12 months. There were 125 unplanned hospitalizations by HF during the follow-up period in 47 (24.5%) patients. Each patient had 1.4±3.64 unplanned HF hospitalizations in the first 12 months.
Primary and secondary endpoints.
| Outcomes | |
| NYHA class at follow-up | |
| Mean±SD | 1.7±0.76 |
| I | 56 (43.4) |
| II | 53 (41.2) |
| III | 18 (14.0) |
| IV | 2 (1.6) |
| NYHA class improvement | 0.4±0.65 |
| HF hospitalizations at 12 months | |
| Total | 32 |
| Mean±SD, per patient | 0.2±0.59 |
| Median [interquartile range], per patient | 0 [0–0] |
| HF hospitalizations during the follow-up | |
| Total | 125 |
| Mean±SD, per patient | 1.4±3.64 |
| Median (interquartile range), per patient | 0 [0–2] |
| CV mortality | 14 (8.8) |
| All-cause mortality | 23 (14.4) |
NYHA: New York Heart Association functional class; SD: standard deviation; HF: heart failure; CV: cardiovascular.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
The groups were well matched in terms of demographics, comorbidities and baseline characteristics (Table 1).
Related to secondary outcomes there were fewer unplanned HF hospitalizations at 12 months in the RM group (P=.02). During the follow-up period there was a tendency toward less unplanned HF hospitalizations in the RM group but without statistical significance (Table 3).
Endpoints of group 1 (remote monitoring) and group 2 (usual standard of care).
| Outcomes | Group 1(n=96) | Group 2(n=96) | P |
|---|---|---|---|
| NYHA class at follow-up | |||
| Mean±SD | 1.7±0.72 | 1.8±0.79 | ns |
| I | 24 (44.4) | 33 (43.4) | ns |
| II | 22 (40.7) | 31 (40.8) | ns |
| III | 8 (14.8) | 10 (13.2) | ns |
| IV | 0 (0.0) | 2 (2.6) | ns |
| NYHA class improvement, mean±SD | 0.4±0.60 | 0.3±0.68 | ns |
| HF hospitalizations at 12 months | |||
| Total | 6 | 26 | .02 |
| Mean±SD, per patient | 0.1±0.25 | 0.2±0.74 | .009 |
| Median (interquartile range), per patient | 0 [0–0] | 0 [0–0] | ns |
| HF hospitalizations during the follow-up | ns | ||
| Total | 40 | 85 | ns |
| Mean±SD, per patient | 1.2±1.93 | 1.6±4.56 | ns |
| Median (interquartile range), per patient | 0 [0–1] | 0 [0–1] | ns |
HF: heart failure; NYHA: New York Heart Association functional class; SD: standard deviation.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
The primary endpoint, CV mortality, occurred in 2 (2.7%) patients in group 1 and 12 (13.8%) in group 2 (hazard ratio (HR) 6.7; 95% confidence interval (CI): 1.46–30.87; P=.004). All-cause mortality occurred in 4 (5.5%) patients in group 1 and 19 (21.8%) in group 2 (HR 5.7; 95% CI: 1.85–17.39; P<0.001) (Table 4). Fig. 2 displays the survival curves for both groups for CV mortality and all-cause mortality, respectively.
Endpoints of group 1 (remote monitoring) and group 2 (usual standard of care).
| Outcomes | Group 1(n=96) | Group 2(n=96) | HR | 95%CI | P by log-rank test |
|---|---|---|---|---|---|
| CV mortality | 2 (2.7) | 12 (13.8) | 6.7 | 1.46–30.87 | .004 |
| All-cause mortality | 4 (5.5) | 19 (21.8) | 5.7 | 1.85–17.39 | <.001 |
95%CI: 95% confidence interval; CV: cardiovascular; HR: hazard ratio.
Data are expressed as no. (%).
From the 96 patients with RM, 51 (53.1%) had active HF fluid retention algorithm and from these 68 alerts were identified with a median duration of 24 (15–40) days. It was possible to assess the motive for the alert and the subsequent intervention in 58 alerts. In all patients in the main island the patient was initially assessed through a telephone consultation. In 13 patients, an in-office visit to the HF clinic was necessary and in seven patients, hospitalization was necessary (in three patients due to HF decompensation and in four due to other motive). Five patients had to be transferred from a peripheral island to the main island (three due to ventricular tachycardia (VT) or ventricular fibrillation (VF) and two due to severe HF decompensation). Seventeen patients from a peripheral island were managed locally in their local health care unit (Table 5).
Management and intervention of the heart failure algorithm alerts.
| Intervention | n (%) |
|---|---|
| Telephone consultation without treatment adjustment | 9 (11.1) |
| Telephone consultation with treatment adjustment | 9 (11.1) |
| In-office visit to the HF clinic | 13 (16.0) |
| Management in the local health care unit | 17 (21.0) |
| HF center hospitalization | 6 (10.3) |
| Transference by airplane from a peripheral island | 5 (6.2) |
| Hospitalization by other motive | 4 (4.9) |
| Total | 58 (100.0) |
HF: heart failure.
Data are expressed as no. (%).
There was a correlation between the number of alerts and total alert duration (days) with the atrial fibrillation (AF) burden (r=0.448, P=0.042, r=0.517, P=0.016, respectively, for number of alerts and total alert duration), number of episodes of VT or VF (r=0.418, P=0.027 and r=0.394, P=0.038, respectively, for number of alerts and total alert duration) and HF hospitalizations (r=0.514, P=0.029 and r=0.624, P=0.006, respectively, for number of alerts and total alert duration) (Table 6).
Correlation between number of alerts, duration of alerts (in days) and clinical outcomes.
| Variables | Number of alerts | Total alert duration (days) | ||
|---|---|---|---|---|
| Spearman correlation coefficient (r) | P | Spearman correlation coefficient (r) | P | |
| Number of HF hospitalizations | 0.514 | .029 | 0.624 | .006 |
| AF burden | 0.448 | .042 | 0.517 | .016 |
| Number of episodes of VT/VF | 0.418 | .027 | 0.394 | .038 |
AF: atrial fibrillation; HF: heart failure; VF: ventricular fibrillation; VT: ventricular tachycardia.
Although promising, the impact of RM in clinical outcomes is still uncertain, as most clinical studies and trials reported conflicting findings. A trial randomizing 335 patients with chronic HF and ICD or cardiac resynchronization defibrillator (CRT-D) to a strategy in which the clinicians and the patient received an alert whenever a higher intrathoracic impedance was detected failed to demonstrate a benefit in reduction of all-cause mortality and HF hospitalizations.10 Another trial randomized 1650 patients with HF and CIED to active RM versus usual standard of care showed no benefit in the outcome death from any cause or unplanned HF hospitalization.12 A randomized trial with 1002 patients with advanced HF also did not show benefit in RM versus standard of care in a composite outcome of all-cause death and cardiovascular (CV) hospitalizations.14 A randomized study, the LIMIT-CHF study, using empirical HF treatment guided by intrathoracic impedance monitoring did not result in a reduction in mortality or HF hospitalizations but improved quality of life.16 Four meta-analyses published between 2009 and 2013 evaluated the effect of RM (including RM of CIEDs and other types of RM as telemonitoring and teleassistance with telephonic consults) in clinical outcomes and reported a reduction in mortality compared to usual standard of care.17–19 A metanalysis by Hindricks et al. (IN-TIME trial) reported a reduction in all-cause mortality and composite endpoint of all-cause mortality and HF hospitalization with RM from CIEDs.20 A meta-analysis combining data from the meta-analysis TRUST, ECOST, and IN-TIME trials reported a reduction in all-cause mortality and composite endpoint of all-cause mortality or HF hospitalizations using RM from CIEDs.21
Some of this controversy can be explained by an ambiguous definition of RM (often but not always associated with CIEDs and in some cases including monitoring of other parameters as vital signs), the type of RM (structured with telephonic support or only telemonitoring), the regularity of transmission of the RM information (prescheduled, on demand or when a pre-defined event occur), the organization of the follow-up team, the feedback speed after an alert is identified and the type of action taken (treatment optimization, assessment in-office or telephonic), the duration of the follow-up, the study design and the patient population.7,22 However, there is very little data regarding the use of RM in remote geographic regions and to the best of our knowledge this is the first study addressing this topic in an insular reality.
It is difficult to find a better scenario than islands to accept all the reported advantages associated with RM. Our hospital is the only central hospital in a nine-island archipelago where most patients only have access to small primary care facilities and a visit to a cardiologist or a cardiology hospitalization requires high cost and long-distance transferences by airplane, sometimes delayed or canceled by meteorological conditions. An acute HF decompensation frequently require activation of an airplane emergency team for differentiated care. RM HF algorithms could potentially avoid these events by identifying patients at risk of decompensation and making possible to address and optimize care before a hospitalization occurs. Besides the elevated costs implicit to a HF hospitalization the costs of transporting these patients are substantial and so, it is our believe that it could be easy to demonstrate significant clinical and economic benefit in such an environment compared with hospitals geographically closer to tertiary centers.
For a successful implementation of a RM program, in any location, it is necessary a structured HF program with organization of a healthcare team with specialized and skilled professionals dedicated to the program and should be customized to address the necessities of the patient population and healthcare center structure. A structured network is essential to identify patients in risk of decompensation and to address and optimize these patients’ conditions promptly, before an hospitalization occurs.
This study was designed to evaluate the effect of RM compared to matched real-world patients that received usual standard of care in a remote insular region. Our findings suggests that RM in this scenario was associated with a lower incidence of CV mortality, all-cause mortality, and unplanned HF hospitalizations during the first 12 months and showed a trend toward less unplanned HF hospitalizations during the follow-up period. Observing the Kaplan–Meier curves, the reduction in all-cause mortality seems to be related with the reduction in CV mortality.
Most patients (87.0%) in our study were from the main island which may be due to the fact that the majority of the population lives in the main island and we cannot exclude a probable referral bias since patients from the peripheral islands tend to be managed conservatively in their local units and do not get enough referrals to a reference center and, as a consequence, to advance therapies as CIEDs. Considering this is the only hospital in the main island and a significant proportion of the patients live in remote areas far from the hospital without easy access to transports we still believe this data to be significant in the context of this special population.
The groups were well matched regarding baseline characteristics and comorbidities. Mean FUP was also similar between groups. NT-proBNP at baseline in G2 was higher but without statistical significance. This could suggest an underlying more decompensated HF in G2. However, the patients baseline characteristics did not differ between groups mainly the mean LVEF and HF etiology suggesting a balanced population of HF patients.
There was a clear correlation between the occurrence and the burden of arrhythmic events as AF, VT and VF, and fluid retention alerts (number and duration of the alerts). The existence of a structured HF management program with an attempted identification of this alerts, and the action taken by the healthcare team may explain the reduction in HF hospitalizations and CV and all-cause mortality in the RM group.
LimitationsOur study is limited by its retrospective nature, data was based on medical records and monitoring platform data, however we believe that being such a unique population it is difficult to implement a randomization. Disease severity was not included in matching criteria, however when comparing the severity through NYHA functional class, NYHA class improvement and LVEF, there was no significant difference between groups. Two different monitoring systems with different parameters and algorithms were used but one system was used more commonly (LATITUD NXT in 76.0%) and HF fluid retention algorithm was not active in all patients. Considering this limitation, the authors chose to use only the number and duration of fluid retention alerts to correlate with clinical outcomes. This study was not designed to address the healthcare team performance when an alert was identified, but to evaluate if a close monitoring of HF patients with CIEDs could improve their clinical outcomes. The treatment was adjusted according to preference of the clinician under the supervision of the HF team in the main island and in line with the international recommendations, but considering this is a remote region, the uptitration of the medical treatment was often done locally in the local health care units and so it was not possible to confirm the dosages and since our data was collected retrospectively some of the data was previous to the widespread use of newer drugs as angiotensin receptor-neprilysin inhibitor (ARNi) and sodium-glucose cotransporter 2 inhibitors (SGLT2i). There was no significant difference in NYHA class improvement in the RM group, which is in line with the fact that all patients had the same type of treatment optimization and so, the symptoms were not expected to be improved using RM. Treatment compliance was also not addressed in this study but may also contribute to the reduction in HF hospitalizations and clinical outcomes. This study did not address economical features, but as mentioned before, a potential economic benefit could probably be present in healthcare associated to HF hospitalizations in an insular reality. The results should be assessed carefully considering this a special insular reality and extrapolation of this results to other contexts should be made with caution and randomized trials are necessary to assess the benefit of RM in other contexts.
ConclusionsRM in the management of HF patients with CIEDs was effective in reducing CV mortality, all-cause mortality and unplanned HF hospitalizations in a remote geographic location as a nine-island archipelago. In the authors perspective RM is an improvement in the follow-up of patients with CIEDs, it is safe and simple to use and may be considered in HF management, especially when geographic limitations are present. The extrapolation of this findings to other contexts is limited due to the unique characteristics of this insular reality (Fig. 3).
Central illustration. RM in in the management of HF patients with CIEDs in an archipelago is effective in reducing CV mortality, all-cause mortality and unplanned HF hospitalizations at 12 months. CIEDs: cardiac implanted electronic devices; CV: cardiovascular; HF: heart failure; RM: remote monitoring.
Self finane.
Ethical considerationsThis study was done in accordance to the standards of research for observational studies defined by the ethical committee of Hospital do Divino Espírito Santo de Ponta Delgada with informed consent of the patients included. The variables of sex have been taken into account in accordance with the SAGER (Sex and Gender Equity in Research) guidelines. Study reporting followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
Statement on the use of artificial intelligenceArtifical intelligence was not used for the development of this work.
Authors’ contributionsM.I. Barradas conceived the idea, collected the data, designed the study, and wrote the paper. F. Duarte and I. Coutinho dos Santos helped in the design of the study and collection of the data. A. Viveiros Monteiro helped with the design and supervised and edited the paper. A. Tavares and D. Martins supervised and reviewed the paper.
Conflicts of interestThe authors have nothing to disclose.
- •
RM is a new paradigm in HF patients with CIEDs and could play a crucial role in identifying earlier signs of HF decompensation and enhancing optimization of care.
- •
The impact of RM in clinical outcomes is still controversial.
- •
In remote geographic regions such as islands, lack of specialized medical facilities and long distances to tertiary centers are often a reality, making HF management even more challenging.
- •
There is very little data regarding the management of HF patients in remote regions of an archipelago.
- •
Our hospital is the only central Hospital in a nine-island archipelago where most patients only have access to small primary care facilities and the transport of each patient requires high cost and long-distance transferences by airplane making HF management challenging.
- •
This study assessed the impact of RM in the management of patients with chronic HF with reduced ejection fraction and CIEDs in the reality of a nine-island archipelago.
- •
RM in our study reduced CV mortality, mortality by all-causes and HF hospitalizations.
- •
RM may be considered in patients with CIEDs particularly in remote regions.