The reduction of low-density lipoprotein cholesterol with lipid lowering therapy has demonstrated to decrease the risk of developing cardiovascular complications during the follow-up in primary and secondary prevention. Unfortunately, a great number of patients do not achieve recommended targets. This is mainly due to an insufficient use of the lipid-lowering therapy currently available. Although healthy life style changes are the basis of the treatment, in many cases this is not enough and it is necessary to add lipid lowering drugs to attain these targets. For these reasons, the current consensus document of the Spanish Society of Cardiology proposes 4 simple and feasible decision-making algorithms according to cardiovascular risk, with the aim of attaining cholesterol goals in the majority of primary prevention patients in a rapid an efficient way.
La reducción del colesterol unido a lipoproteínas de baja densidad con el tratamiento hipolipidemiante ha demostrado disminuir el riesgo de complicaciones cardiovasculares durante el seguimiento, tanto en prevención primaria como secundaria. Desafortunadamente, un gran número de pacientes no logran los objetivos de control recomendados. Esto se debe, sobre todo, a un uso insuficiente de las terapias hipolipidemiantes disponibles. Aunque la base del tratamiento lo constituyen los cambios hacia un estilo de vida más saludable, en numerosas ocasiones esto no es suficiente y es necesario añadir un tratamiento hipolipidemiante para alcanzar estos objetivos. Por estos motivos, en el presente documento de consenso de la Sociedad Española de Cardiología se proponen 4 algoritmos de abordaje en función del riesgo cardiovascular de los pacientes, sencillos y fácilmente aplicables, con el objetivo de lograr que la mayoría de los pacientes en prevención primaria cumplan los objetivos de control de colesterol de manera rápida y eficiente.
In the last few years, mortality from cardiovascular (CV) disease has decreased, but it continues to be the first cause of death in Spain.1 Multiple evidence demonstrates that high cholesterol levels, particularly low-density lipoprotein cholesterol (LDLc) and apolipoprotein B-containing proteins, not only increase the risk of developing atherosclerotic CV disease, but also constitute the etiopathogenic basis of atherosclerosis.2–4
Previous studies have shown that better control of CV risk factors, especially hypertension and dyslipidemia, substantially reduces age-adjusted mortality from ischemic heart disease.5,6 Indeed, reducing LDLc through lipid-lowering therapy is associated with a significant decrease in major CV events in both primary and secondary prevention, independently of age.7,8 Moreover, this benefit may not depend on specific lipid-lowering therapy but on the effectiveness of the drug in reducing LDLc.9
Unfortunately, LDLc control, both in primary and secondary prevention, is clearly deficient, not only in Spain but also in other countries,10–13 which is associated with a higher risk of CV complications and substantially increases health care expenditure.14,15 This situation has become even more important during the pandemic due to severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), during which in-hospital mortality has increased among patients with a myocardial infarction, possibly facilitated—at least in part—by limited health care and worse CV risk factor control.16 Moreover, it has been reported that, in patients hospitalized for the disease caused by SARS-CoV-2 (COVID-19), statin therapy prior to hospital admission was associated with a lower risk of severe disease and lower mortality.17,18
In France, treatment algorithms were published to optimize lipid-lowering therapy after an acute coronary syndrome (ACS).19 The application of these algorithms between February 2017 and September 2018 resulted in 50% of the patients achieving LDLc levels <55mg/dL and in 66% achieving a decrease in LDLc of at least 50%, and this improvement persisted at 5 months of follow-up.20 More recently, The Spanish Society of Cardiology (SEC) has proposed 3 simple and practical algorithms to optimize lipid-lowering therapy in the entire spectrum of patients with ischemic heart disease (acute, chronic with extreme CV risk, and chronic with very high CV risk), with the dual aim of improving values of LDLc control as quickly as possible, since the benefit not only depends on the magnitude of the LDLc reduction but also on the time in which the reduction is achieved.21 Moreover, to aid implementation of the algorithm, an open-access algorithm has been created, “Control lipídico” (in English, “Lipid control”).22 Based on this consensus, a study that included 2285 patients with ACS consecutively admitted to a tertiary hospital in Murcia found that approximately half the patients should receive lipid-lowering therapy with high-intensity statins (LCLc reduction≥50%) plus ezetimibe on hospital discharge.23
Likewise, early lipid-lowering therapy in primary prevention in young patients has been reported to result in a long-term reduction in CV risk,24,25 emphasizing the need for the early achievement of LDLc targets in primary prevention as well. The 2019 European guidelines for dyslipidemia propose a stepwise treatment strategy, which could delay the achievement of LDLc objectives, especially in patients with the highest CV risk.4 Recently, the American Association of Clinical Endocrinologists and the American College of Endocrinology have proposed several algorithms for the treatment of dyslipidemia, depending on CV risk.26 However, these associations use a scale for stratifying CV risk that differs from the European scale, and consequently its application is difficult in our environment. Therefore, there is a need to create specific algorithms in primary prevention to improve the approach to these patients in our milieu both rapidly and effectively. The present consensus document of the SEC describes several algorithms for the optimization of lipid-lowering therapy in primary prevention, depending on CV risk. The document is the result of the participation of experts in dyslipidemia from distinct specialties (cardiology, internal medicine, and primary care).
Cardiovascular risk stratificationThe guidelines of the European Society of Cardiology and the European Society of Atherosclerosis for the treatment of patients with dyslipidemia recommend CV risk stratification through the Systematic Coronary Risk Estimation (SCORE) chart which, in the case of Spain, corresponds to low-risk European regions.4
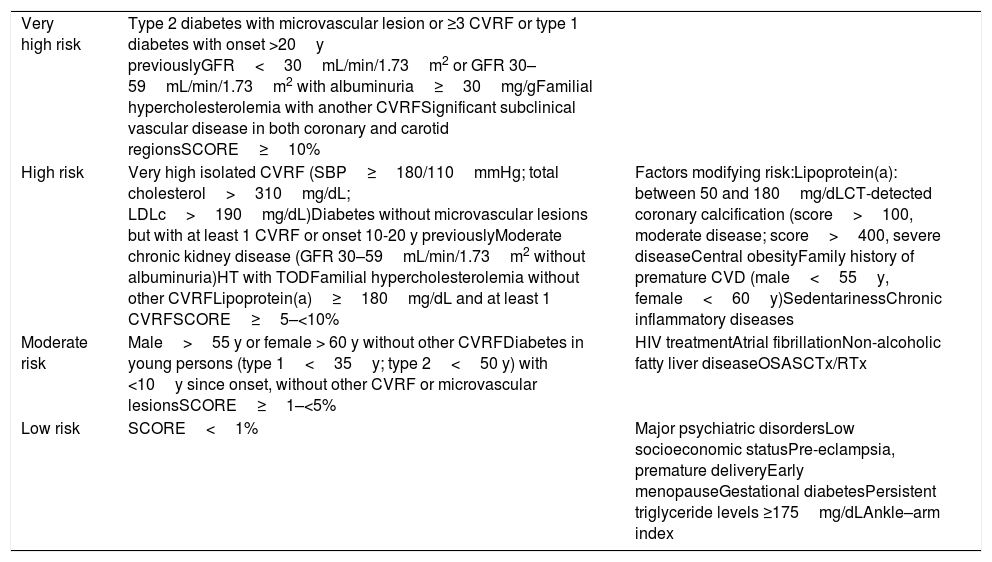
Considering the information available from various studies, in the present consensus document, we decided to add certain patient profiles to the CV risk chart proposed by the European guidelines on dyslipidemia. In chronic kidney disease, CV risk is increased not only by a reduced glomerular filtration rate but also by the presence of albuminuria.27 Consequently, we believe that patients with a glomerular filtration rate of 30-59mL/min/1.73m2 and albuminuria≥30mg/g have very high CV risk. In addition, in patients with hypertension, the presence of associated organ damage (left ventricular hypertrophy and oligoalbuminuria) confers an elevated CV risk.28 High lipoprotein(a)—Lp(a)—levels are associated with an increased risk of CV events. In agreement with the European guidelines on dyslipidemia, persons with Lp(a) levels >180mg/dL have a similar risk of developing atherosclerotic CV disease to patients with heterozygous familial hypercholesterolemia, and consequently persons with Lp(a) levels >180mg/dL associated with at least 1 CV risk factor should be considered as being at elevated CV risk.4 In agreement with the SCORE charts for low-risk countries, non-smoking men, with blood pressure 120mmHg and total cholesterol 155mg/dL, in the absence of other risk factors and without CV disease, would have a SCORE risk of 1% at the age of 55 years. In women, this profile corresponds to those aged 60 years.4 For this reason, it has been specified that men >55 years and women >60 years without other risk factors for CV disease should be considered as being at moderate risk. Table 1 specifies the factors that should be considered as increasing risk.4,24,29,30
Cardiovascular risk stratification in patients in primary prevention.
| Very high risk | Type 2 diabetes with microvascular lesion or ≥3 CVRF or type 1 diabetes with onset >20y previouslyGFR<30mL/min/1.73m2 or GFR 30–59mL/min/1.73m2 with albuminuria≥30mg/gFamilial hypercholesterolemia with another CVRFSignificant subclinical vascular disease in both coronary and carotid regionsSCORE≥10% | |
| High risk | Very high isolated CVRF (SBP≥180/110mmHg; total cholesterol>310mg/dL; LDLc>190mg/dL)Diabetes without microvascular lesions but with at least 1 CVRF or onset 10-20 y previouslyModerate chronic kidney disease (GFR 30–59mL/min/1.73m2 without albuminuria)HT with TODFamilial hypercholesterolemia without other CVRFLipoprotein(a)≥180mg/dL and at least 1 CVRFSCORE≥5–<10% | Factors modifying risk:Lipoprotein(a): between 50 and 180mg/dLCT-detected coronary calcification (score>100, moderate disease; score>400, severe diseaseCentral obesityFamily history of premature CVD (male<55y, female<60y)SedentarinessChronic inflammatory diseases |
| Moderate risk | Male>55 y or female > 60 y without other CVRFDiabetes in young persons (type 1<35y; type 2<50 y) with <10y since onset, without other CVRF or microvascular lesionsSCORE≥1–<5% | HIV treatmentAtrial fibrillationNon-alcoholic fatty liver diseaseOSASCTx/RTx |
| Low risk | SCORE<1% | Major psychiatric disordersLow socioeconomic statusPre-eclampsia, premature deliveryEarly menopauseGestational diabetesPersistent triglyceride levels ≥175mg/dLAnkle–arm index |
CTx, chemotherapy; CVD, cardiovascular disease; CVRF, cardiovascular risk factors; GFR, glomerular filtration rate; HIV, human immunodeficiency virus; HT0, hypertension; LDLc, low-density lipoprotein cholesterol; OSAS, obstructive sleep apnea syndrome; SBP, systolic blood pressure; TOD, target organ damage; RTx, radiotherapy.
Table based on Mach et al.,4 Pencina et al.,24 López-Fernández et al.,29 and Arnett et al.30
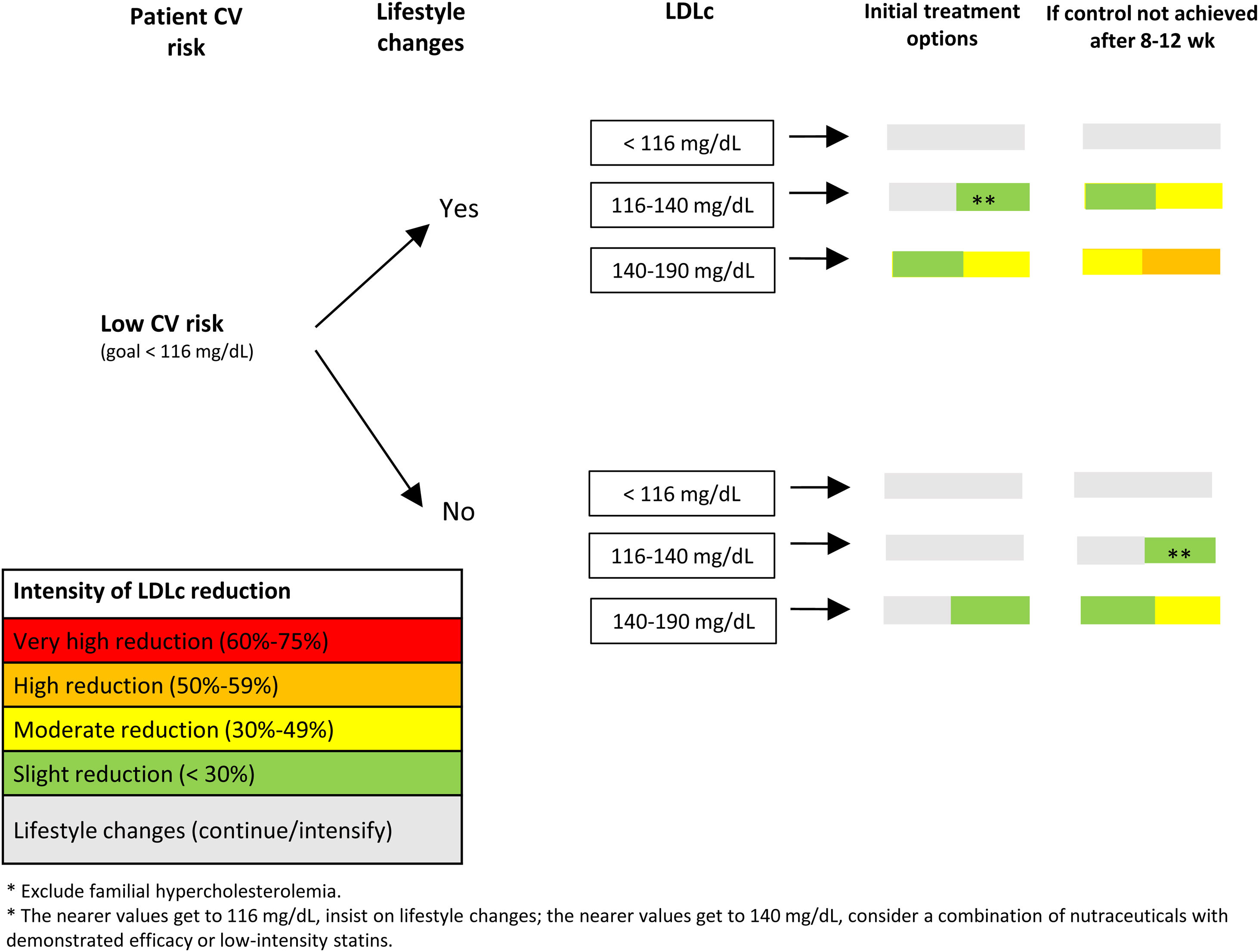
In agreement with the European guidelines on dyslipidemia, we propose goals of LDLc<55mg/dL and a reduction of at least 50% in persons at very high CV risk, LDLc<70mg/dL and a reduction of at least 50% in individuals at high CV risk, LDLc<100mg/dL in persons at moderate CV risk, and LDLc<116mg/dL in those at low CV risk.4
LDLc-lowering treatmentsAll patients with dyslipidemia should be encouraged to make lifestyle changes, including following the Mediterranean diet, abstaining from smoking, taking physical exercise, and losing weight if they are overweight or obese, in addition to controlling the remaining CV risk factors.4
Dietary recommendations advise a healthy diet, with low saturated fat intake and rich in wholemeal products, fruit, vegetables, and fish. The Mediterranean diet is recommended, among others. The PREDIMED trial demonstrated that the Mediterranean diet supplemented with extra virgin olive oil and dried fruits can significantly reduce (almost by 30%) the rate of major CV events compared with a low-fat diet.31 However, the main problem of this enriched diet is follow-up and long-term adherence.32 Therefore, its ability to reduce LDLc depends on individuals’ persistence in following it.
In addition to making lifestyle changes, patients at high or very high CV risk should be started on lipid-lowering therapy to achieve LDLc goals. In contrast, patients with low or moderate CV risk should begin with lifestyle changes and, if these prove insufficient, lipid-lowering therapy should be considered. In these patients, the delay in starting lipid-lowering therapy depends on their adherence to diet and physical exercise, their baseline LDLc value (the higher the LDLc level, the shorter the delay), the patient's CV risk, and whether there are other factors that could increase CV risk.4 For example, in patients with low CV risk, who are usually young, the need for lipid-lowering therapy should be discussed, stressing the advisability of achieving a healthy lifestyle, especially in patients whose cholesterol levels are not very high, since these measures can be sufficient in a substantial number of individuals.
With regard to food supplements, the European guidelines on dyslipidemia state that the available evidence on the effect of nutraceuticals on lipid profile is incomplete. An important nutraceutical, among others, is red yeast rice (monacolin K), which has been demonstrated to be effective in reducing LDLc. Nevertheless, the safest dose is monacolin K 3mg since the highest dose (10mg) has the same adverse effects as statins. Nutraceuticals combining several natural components are those with the highest efficacy.33 Among the various nutraceuticals, those supported by the most robust scientific evidence should be used.33,34 Their main indication is in patients with low CV risk, in whom lifestyle modification is insufficient to achieve LDLc goals and the option of statin use is not preferred (patient preference, adverse effects of statins, etc.).32
Statins reduce cholesterol synthesis by the liver through competitive inhibition of 3-hydroxy-3-methyl-glutaryl-CoA reductase. Although all statins share the same mechanism, not all reduce LDLc with the same intensity. High-intensity statins reduce LDLc by about 50% from baseline levels, moderate-intensity statins lower LDLc by 30% to 50%, and low intensity statins decrease LDLc by less than 30%.4,35 Nevertheless, there is wide interindividual variability in response to statin therapy.4 In addition, the risk of adverse effects, mainly hepatic (transaminase elevation) and muscular (myalgia, creatine-phosphokinase elevation), also differs among statins. Statins with the least risk of interactions are those with the lowest possibility of producing adverse effects. Thus, statins not metabolized by cytochrome P450 (pravastatin, rosuvastatin, and pitavastatin) have a lower risk of interactions.4 In primary prevention, statins have been demonstrated to improve CV prognosis. For example, in the JUPITER trial, which included approximately 18000 patients without prior CV disease, with a LDLc≤130mg/dL and high-sensitivity C-reactive protein≥2.0mg/L, treatment with rosuvastatin 20mg/d compared with placebo was associated with a significant reduction in the risk of major CV events of 47%, and the risk of all-cause mortality was reduced by 20% with only 1.9 years of follow-up.36 Therefore, statins are the treatment of choice in primary prevention in persons not achieving LDLc goals after making lifestyle changes. The statin chosen should be that producing the required LDLc reduction, with the best safety profile.
Ezetimibe inhibits the absorption of biliary and dietary cholesterol from the intestine but without affecting the absorption of fat-soluble nutrients.4 In monotherapy, ezetimibe 10mg/d reduces LDLc by around 15% to 22%, with substantial interindividual variability.37 When added to statin therapy, ezetimibe produces an additional decrease in LDLc of around 15%. However, to reduce LDLc, it is more effective to add ezetimibe to statin therapy than to double the statin dose.38 In the IMPROVE-IT trial, conducted in patients admitted for an ACS, the combination of simvastatin 40mg with ezetimibe 10mg (with a median LDLc level during follow-up of 55mg/dL) was associated with a significant decrease in the composite endpoint of CV events vs simvastatin 40mg monotherapy (median LDLc level of 70mg/dL during follow-up), with no increase in the risk of adverse events.39
Bempedoic acid inhibits cholesterol synthesis by inhibiting adenosine triphosphate citrate lyase, a cytosolic enzyme that acts before 3-hydroxy-3-methyl-glutaryl coenzyme reductase in the cholesterol biosynthetic pathway.40 Compared with placebo, bempedoic acid is able to reduce LDLc by between 27.4% and 28.5%, with additional decreases when used in combination with ezetimibe.4,41 Although currently ongoing studies will determine its ability to reduce CV events, a combined analysis of the available evidence showed that treatment with bempedoic acid could reduce the risk of major CV events (hazard ratio, 0.83; 95% confidence interval, 0.57–1.21).42 Bempedoic acid is indicated in combination with a statin or with a statin and other lipid-lowering treatments in patients not achieving target LDLc levels, or alone or in combination with other lipid-lowering treatments in statin-intolerant patients.4,41
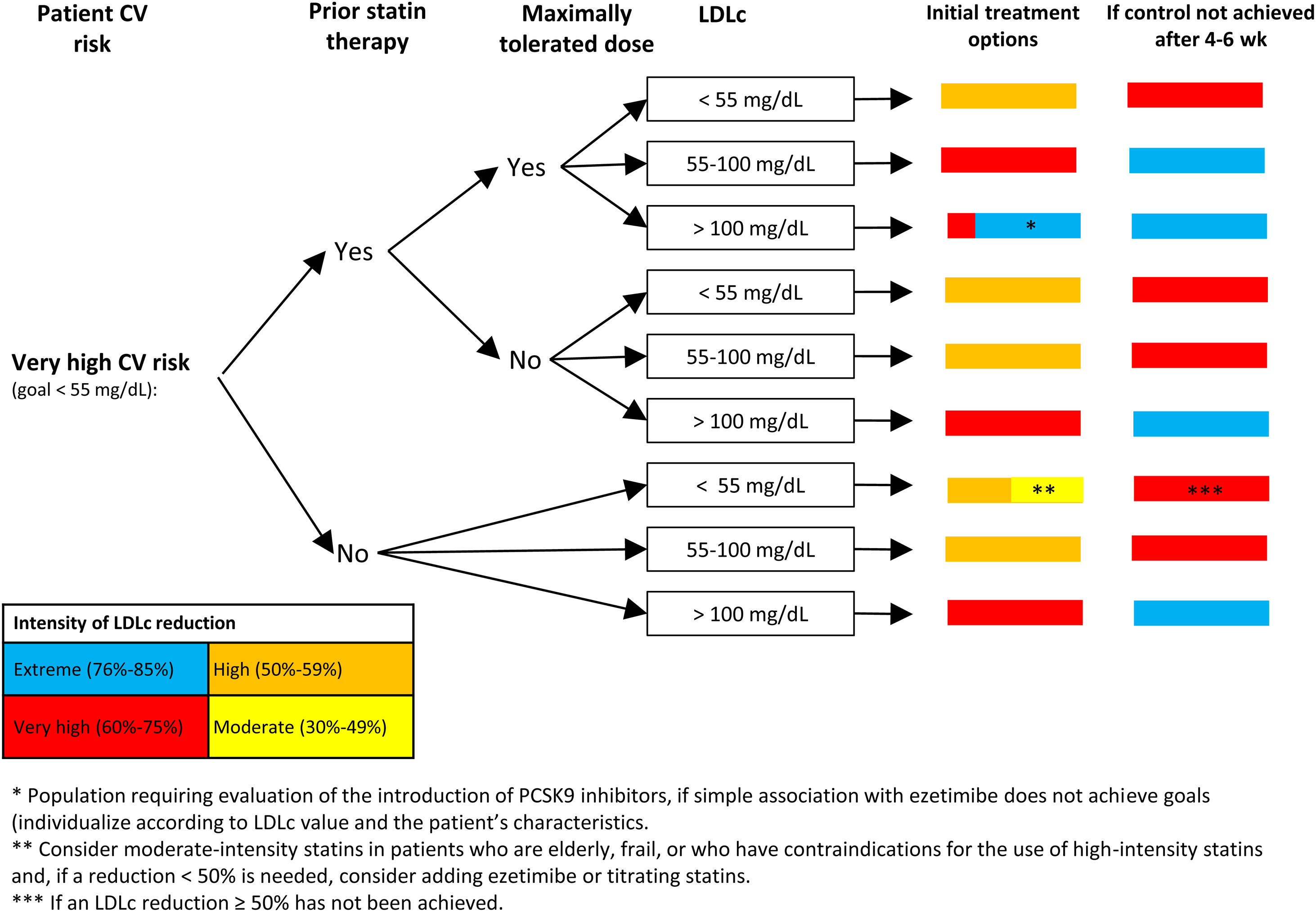
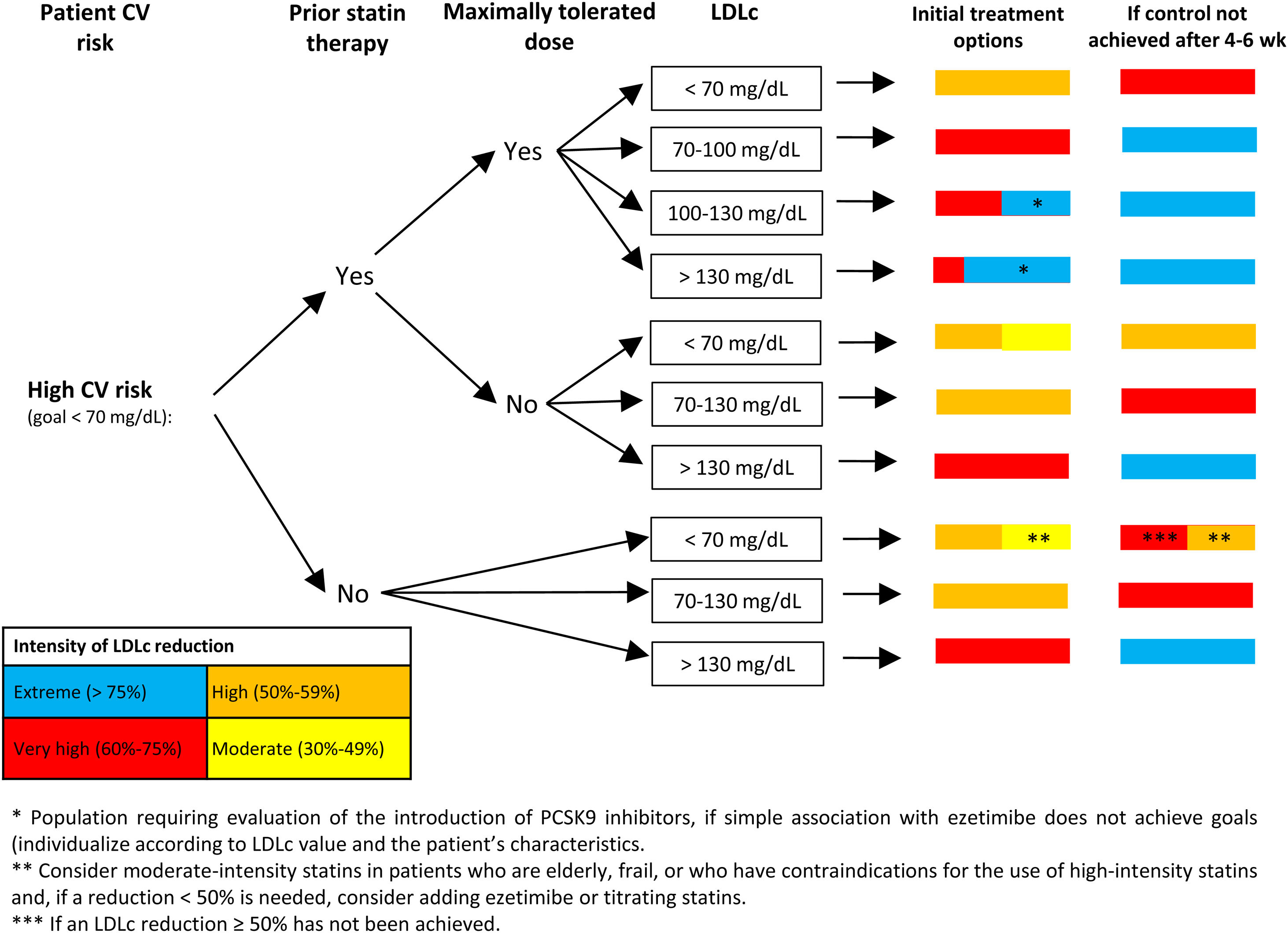
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) markedly reduce LDLc. In the evolocumab arm of the FOURIER trial, which included patients with atherosclerotic CV disease, the mean LDLc value was 30mg/dL, while in the alirocumab arm of the ODYSSEY OUTCOMES trial, which included patients with an ACS in the past 12 months, the initial reduction in LDLc was 38mg/dL. In both trials, the addition of PCSK9i to the background lipid-lowering therapy was associated with a significant 15% reduction in the primary endpoint.43,44 The European guidelines on dyslipidemia recommend the addition of PCSK9i inhibitors to treatment if LDLc goals are not achieved with statins at the maximum tolerated dose and ezetimibe in secondary prevention (class 1 recommendation, level of evidence A), familial hypercholesterolemia and very high CV risk (class 1 recommendation, level of evidence C). PCSK9i can also be considered in primary prevention in individuals with very high CV risk without familial hypercholesterolemia (class IIB recommendation, level of evidence C).5 Recently, the Spanish Society of Atherosclerosis has published recommendations on the use of PCSK9i aimed at patients who could obtain greater benefit from these drugs (Fig. 1 and Fig. 2).45
Algorithm for the approach to lipid-lowering treatment in patients with very high CV risk. Recommendations of the Spanish Society of Atherosclerosis (SEA): use of PCSK9 inhibitors in primary prevention: homozygous familial hypercholesterolemia and LDLc>100mg/dL; heterozygous familial hypercholesterolemia with ≥4 associated risk factors and LDLc>130mg/dL or diabetes and LDLc>100mg/dL; CKD≥3b (no dialysis) with diabetes and LDLc>130mg/dL.45 According to the 2019 European guidelines on dyslipidemia,4 in patients in primary prevention with very high risk and no familial hypercholesterolemia, the addition of a PCSK9 inhibitor can be considered when the LDLc target is not achieved with the combination of maximum tolerated dose and ezetimibe. In patients with familial hypercholesterolemia and very high risk who do not achieve the LDLc goal with the combination of maximum tolerated statin dose and ezetimibe, addition of a PCSK9 inhibitor is recommended. CKD, chronic kidney disease; CV, cardiovascular; LDLc, low-density lipoprotein cholesterol.
Figure adapted with permission from Escobar et al.21
Algorithm for the approach to lipid-lowering treatment in patients with high cardiovascular risk. Recommendations of the Spanish Society of Atherosclerosis (SEA): use of PCSK9 inhibitors in primary prevention: homozygous familial hypercholesterolemia and LDLc>100mg/dL; heterozygous familial hypercholesterolemia with ≥4 associated risk factors and LDLc>130mg/dL or diabetes and LDLc>100mg/dL; CKD≥3b (no dialysis) with diabetes and LDLc>130mg/dL.45 The 2019 European guidelines on dyslipidemia4 do not recommend the use of PCSK9 inhibitors in patients at high cardiovascular risk in primary prevention. CKD, chronic kidney disease; CV, cardiovascular; LDLc, low-density lipoprotein cholesterol.
Figure adapted with permission from Escobar et al.21
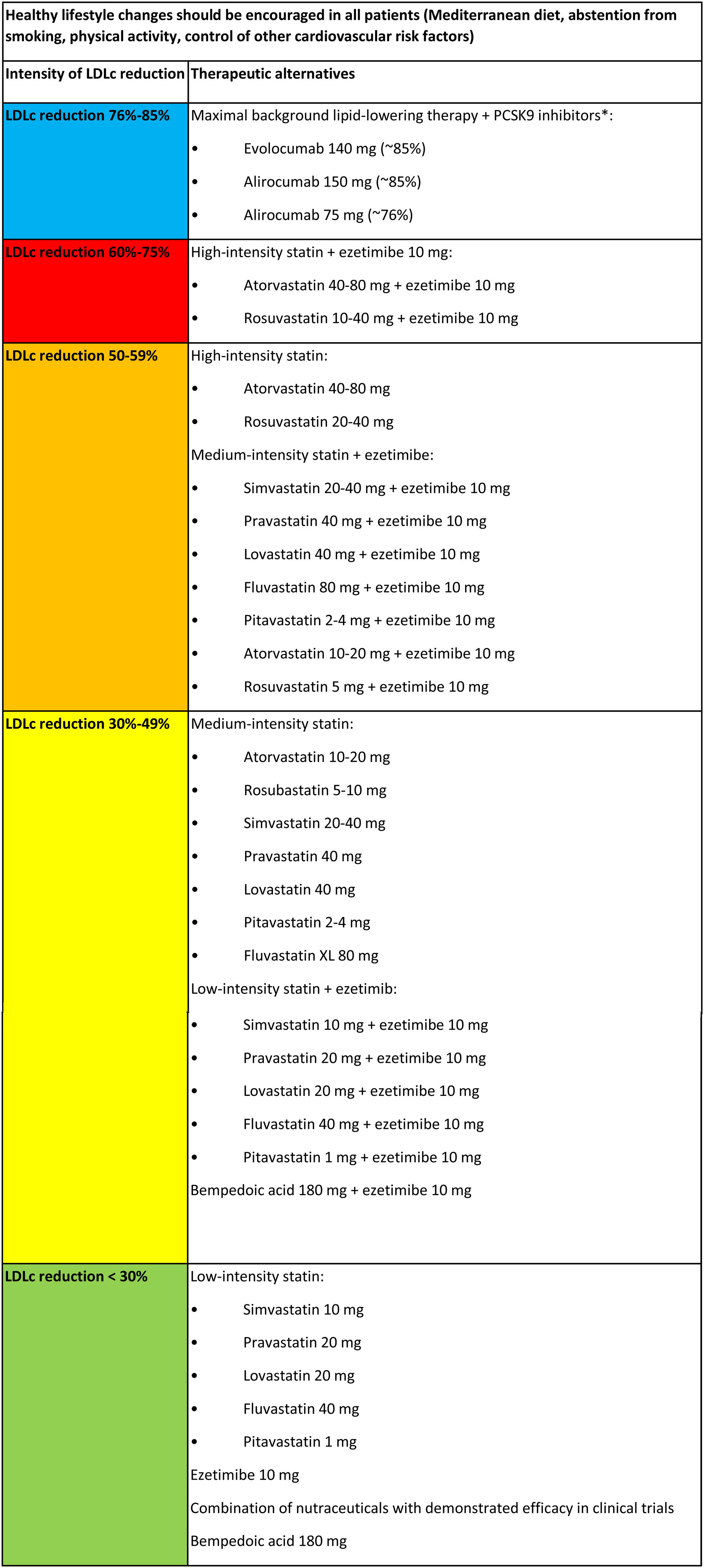
Fig. 3 summarizes the various lipid-lowering therapies, classified according to their ability to reduce LDLc in monotherapy or in combination.
Classification of lipid-lowering therapy according to intensity of LDLc reduction.
CVRF, cardiovascular risk factors; LDLc, low-density lipoprotein cholesterol.
Figure adapted with permission from Escobar et al.21 and based on Mach et al.,4 Masana et al.,35 and Saeed et al.40
* In general, starting with maximal doses of PCSK9 inhibitors is recommended to achieve the maximum reduction possible from the outset.
Currently, new therapeutic options are under study that, in the future, will expand the number of currently availably lipid-lowering treatments. Thus, inclisiran, a small chemically synthesized interfering RNA molecule administered every 6 months, reduces PCSK9 synthesis in the liver, reducing LCLc by around 50% to 55%.46 Drugs specifically designed to reduce Lp(a) are also under development. The most promising is pelacarsen, which is targeted to LPA mRNA and is administered every 2 to 4 weeks subcutaneously.47
Algorithm interpretationThis document presents 4 scenarios with a different approach according to patients’ CV risk. Lifestyle modifications are recommended in all scenarios and in patients with high or very high CV risk, in addition to initial drug therapy.
In patients with very high CV risk (Fig. 1), the target LDLc level is <55mg/dL and a reduction of at least 50% in LDLc. As shown in Fig. 1, the treatment strategy depends on whether the patient is receiving statin therapy and whether these are prescribed at the maximum tolerated dose. Subsequently, depending on LDLc levels, treatment recommendations are made when attending the patient, according to the color code in Fig. 3, depending on the reduction needed in LDLc. Patients are reassessed in 4 to 6 weeks and lipid-lowering therapy is intensified, if necessary. This algorithm is similar to that proposed in the consensus document on secondary prevention of the SEC.19 In the case of patients in primary prevention, the evidence on the benefit of PCSK9i is less solid, especially in those without familial hypercholesterolemia.45 Interpretation of the algorithm is similar for patients with high CV risk (Fig. 2): an LDLc target of <70mg/dL and an LDLc reduction of at least 50%. In patients with high CV risk, the recommendations for PCSK9i should be more restrictive.
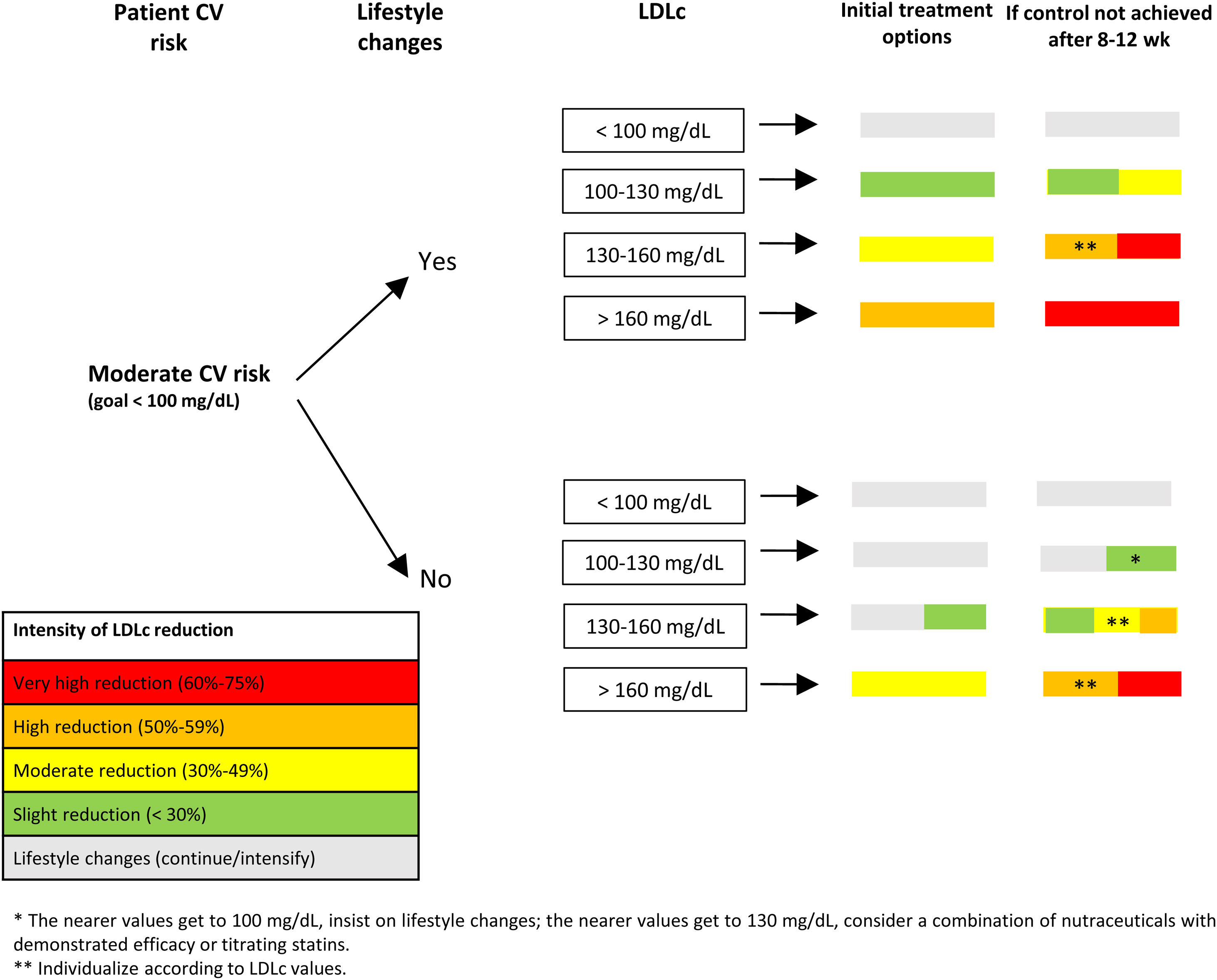
In patients with moderate (Fig. 4) or low (Fig. 5) CV risk, it is important to stress the need for healthy lifestyle habits and to start lipid-lowering treatment if these are insufficient and LDLc levels are above recommended goals. The interval before starting lipid-lowering therapy will depend on the patient's adherence to treatment, CV risk, and LDLc levels. Subsequently, and following the colors in Fig. 3, various treatment options can be chosen, depending on the patient's CV risk and LDLc level. In both cases, the patient should be reassessed at 8 to12 weeks.
Despite the demonstrated benefits of LDLc reduction in primary prevention, a large number of patients will not achieve target goals, representing an increase in the risk of CV complications. Although lifestyle changes form the basis of treatment, they are often insufficient and lipid-lowering therapy will be needed to achieve target levels. The 4 treatment algorithms presented in this document are simple to apply and aim to aid the therapeutic approach to patients with dyslipidemia in primary prevention.
FundingThis work was supported by an unconditional grant from Mylan and Almirall, which did not influence the content of the manuscript in any way.
Authors’ contributionsAll authors contributed substantially to the conception, design, drafting, and review of the manuscript. Va. Barrios and C. Escobar also coordinated the study.
Conflicts of interestV. Barrios and J. Cosín-Sales have received grants, speaker, and consultant fees from Sanofi, Almirall, MSD and Mylan. C. Escobar has received grants, speaker and consultant fees from Amgen, Sanofi, MSD, Mylan, Servier, Ferrer, Daiichi-Sankyo, and Novartis. V. Arrarte and M. Seoane have received grants, speaker and consultant fees from MSD, Amgen, Sanofi, Rovi, and Almirall. R. Campuzano has received grants, speaker and consultant fees from Amgen, Sanofi, MSD, Mylan, Servier, Ferrer, Daiichi-Sankyo, and Novartis. J.M. Gámez has received grants, speaker and consultant fees from Sanofi, Amgen, Novartis, Mylan and Almirall. C. Guijarro has received grants, speaker and consultant fees from Amgen, Sanofi, Daiichi Sankyo, Ferrer, Pfizer, Laboratorios Rubió and MSD. J.M. Mostaza has received grants, speaker or consultant fees from Amgen, Sanofi, Novartis, and Daiichi-Sankyo. P. Valdivielso has received grant, speaker and consultant fees from Ferrer, Amgen, Sanofi, Novartis, Daiichi-Sankyo, MSD, and Amarin. Á. Cequier has received grants, speaker, and consultant fees from Abbott Vascular, Biosensors, Boston Scientific, Medtronic, Biomenco, Cordis, Orbus Neich, the Spanish Society of Cardiology, Ferrer International, Terumo, Sanofi, Novo Nordisk, Amgen, Bayer, Boehringer Ingelheim, and Daiichi-Sankyo. M. Anguita, I. Egocheaga, J.C. Obaya and V. Pallarés-Carratalá have no conflicts of interest to declare.
Abbreviations: ACS, acute coronary syndrome; CV, cardiovascular; LDLc. low-density lipoprotein cholesterol; Lp(a), lipoprotein(a).
This article is an English translation of the article “Recomendaciones para mejorar el control lipídico en pacientes en prevención primaria. Documento de consenso de la Sociedad Española de Cardiología” published in REC Cardioclinics. 2021;56:118–128. The Spanish version of this document is available at doi:10.1016/j.rccl.2021.02.006