The concurrence of cancer and ischemic heart disease is a controversial issue, given the confluence of risk factors for both diseases. The aim of this study is to analyze the incidence, predictors, and prognosis of new-onset cancer after an acute coronary syndrome (ACS).
MethodsRetrospective multicenter registry that includes 3867 consecutive patients discharged for ACS without a known history of cancer, from 2011 to 2015. The association between clinical variables and cancer occurrence was assessed by Fine-Gray proportional hazards regression analysis, and its prognostic impact was evaluated using Cox and Fine-Gray regression models.
ResultsThe cumulative incidence of cancer was 2.47 per 100 patient/years. Four independent predictors of follow-up cancer were identified: age, male sex, smoking, and human immunodeficiency virus infection. We have constructed a simple risk score, based on the coefficients of these variables in the final multivariable model. Using this score, we stratified our population according to the risk of developing cancer: low (1.03% per year), moderate (2.35% per year), and high-risk (10.51% per year) groups. Post-discharge cancer was independently associated with an increased risk of non-cardiovascular death (HR, 17.65; 95%CI, 10.47–29.76) and bleeding (sHR, 2.54; 95%CI, 1.21–5.34), but not with reinfarction (sHR, 0.50; 95%CI, 0.16–1.56).
ConclusionsCancer occurrence after hospital discharge for ACS is relatively low, but it could be predicted by the combination of 4 easily available clinical risk factors: age, male sex, smoking, and human immunodeficiency virus infection. Post-discharge cancer is associated with a higher risk of subsequent death and bleeding events.
La simultaneidad de cáncer y cardiopatía isquémica es un tema controvertido, dada la confluencia de factores de riesgo entre ambas enfermedades. Con este estudio se pretende analizar la incidencia, los factores predictores y el pronóstico del cáncer de novo tras el alta hospitalaria por síndrome coronario agudo (SCA).
MétodosSe trata de un registro multicéntrico retrospectivo que incluyó 3.867 pacientes dados de alta tras el SCA y sin historia previa de cáncer, de forma consecutiva desde 2011 hasta 2015. La asociación entre las variables basales y cáncer se estudió mediante un análisis de regresión de Fine-Gray, y su impacto pronóstico se evaluó mediante modelos de regresión de Cox y de competencia de riesgo (Fine-Gray).
ResultadosLa incidencia acumulada de cáncer fue de 2,47 por 100 pacientes/año. Se identificaron 4 factores de riesgo: edad, sexo masculino, tabaquismo e infección por el virus de la inmunodeficiencia humana. De esta forma se construyó una escala de riesgo simplificada basada en los coeficientes de estas variables en el modelo multivariado final. Con esta puntuación se estratificó nuestra población en función del riesgo de desarrollar cáncer: riesgo bajo (1,03% por año), moderado (2,35% por año) y alto (10,51% por año). El desarrollo de cáncer tras el alta se asoció de forma independiente con un riesgo aumentado de muerte de causa no cardiovascular (HR=17,65; IC95%, 10,47-29,76) y hemorragia (sHR=2,54; IC95%, 1,21-5,34), pero no con reinfarto (sHR=0,50; IC95%, 0,16-1,56).
ConclusionesLa aparición de cáncer tras el ingreso hospitalario por SCA es relativamente baja, pero se puede predecir a partir de la combinación de 4 factores de riesgo fácilmente disponibles en la práctica clínica: edad, sexo masculino, tabaquismo e infección por el virus de la inmunodeficiencia humana. El cáncer de novo se asoció con mayor riesgo consecuente de muerte no cardiovascular y hemorragia.
Cardiovascular disease and cancer are the 2 leading causes of death worldwide. Although commonly thought of as 2 separate disease entities, they present various similarities and possible interactions, including a number of similar risk factors (eg, age, smoking, obesity), suggesting a shared biology for which there is emerging evidence.1,2 Accordingly, an increased risk of cancer amongst patients with acute myocardial infarction (AMI) has been reported.3–5 However, the association between ischemic heart disease and cancer raises several concerns, such as controversial issues regarding the effects of specific cardiovascular medication (antiplatelet drugs, angiotensin-receptor blockers, or statins).6–14 Currently, there are very few contemporary studies from real-life patients analyzing the variables associated with cancer after an acute coronary syndrome (ACS).15 Therefore, identifying cancer predictors in patients with ACS is imperative (or necessary, or justified) so as to identify subjects with a high risk of cancer and pinpoint modifiable risk factors susceptible of intervention in order to reduce cancer incidence in these patients.
This study aims to analyze the incidence, predictors and impact prognosis of post-discharge cancer after an ACS in a contemporary real-world setting.
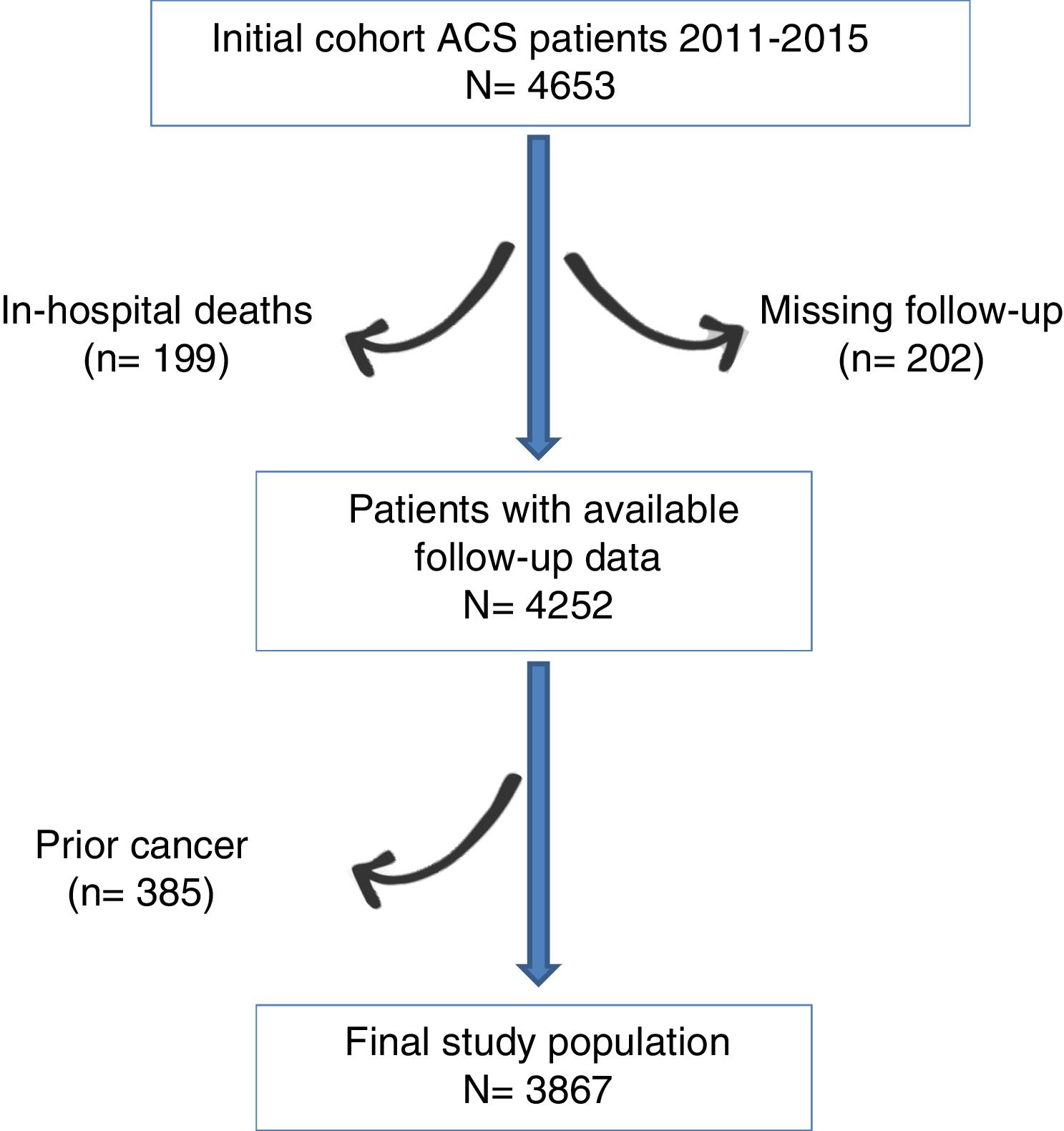
MethodsPatient populationThe data analyzed in this study were obtained from a merged retrospective clinical registry including all patients consecutively admitted to the cardiology department of two tertiary hospitals from 2011, January to 2015, December, with the primary and definitive diagnosis of ACS, underwent coronary angiography (n=4653). Patients who died in hospital (n=199; 4.3%) and those with no data about vital status and/or on cancer development during follow-up (n=202; 4.5%) were excluded. Since our objective was to study the incidence and predictor of “de novo” cancer, we excluded those patients with prior diagnosis of cancer at hospital discharge (n=385). Thus, the final cohort of the present study consisted of 3867 patients. The flowchart can be seen in Fig. 1.
Data on demographic and clinical characteristics, complementary test results, echocardiographic and angiographic parameters, in-hospital events, as well as treatment at discharge, were collected in detail by trained cardiologists.
The present study complied with the Declaration of Helsinki and the normative of the local ethics committee.
Study endpoint, definitions, and follow-upThe ascertainment of cancer status and death during follow-up was carried out between December of 2016 and January of 2017 by trained physicians. We reviewed the electronic medical registry and all the medical attendances and hospital records.
Follow-up “de novo” cancer was defined as non-benign neoplasm events after hospital discharge, being the diagnosis based on pathologic data and clinical information. The first priority to confirm the diagnosis was a definitive pathologic diagnosis from a pathology report. If there was no definitive pathologic diagnosis available, then the non-benign neoplasm diagnosis was established by the best pathologic data available, clinical information (such as the anatomic distribution of the neoplasm from imaging reports), and the consensus opinion of the physicians that adjudicated the events. For verified non-benign neoplasm events, the dates of initial detection and histological diagnosis and the anatomic/tissue location of the malignant neoplasm were determined. Cancer types were classified by anatomic and system primary involvement.
Reinfarction was defined as the elevation of troponins associated with changes in the electrocardiogram or typical chest pain after admission for the main event. Bleeding events were classified according to Bleeding Academic Research Consortium (BARC) criteria.16 Due to the retrospective nature of our study and the difficulty for the accurate identification of all BARC type 1 bleeding; this type of bleeding was not included in the present study. Therefore, only the BARC 3–5 bleeding events were analyzed.
Statistical analysisCategorical variables were presented as frequency values, and continuous variables were presented as mean±standard deviation. Differences in baseline characteristics were compared using the Student t test for continuous variables and the chi-square test for categorical variables. The rate of de novo post-discharge cancer was reported as the cumulative incidence function. To assess the association between clinical variables and the development of de novo cancer, we performed a Fine-Gray proportional hazards regression analysis, accounting for death as a competing episode. The variables associated with post-discharge cancer in the univariate analysis (P<.05) were included in a multivariate model, forcing the inclusion of age, sex, and smoking, regardless of the P-value. The c-index was calculated for each final model to evaluate the ability of the model to predict post-discharge cancer admission, using the c-index function of the R package “pec”. The adjusted hazards were expressed as subhazard ratios (sHR) with their corresponding 95% confidence intervals (95%CI).
We developed a simple scoring system basing on the coefficients of the independent predictors retained in the final multivariate model. This was done in order to make it easier for clinicians to estimate risk obtained from the multivariable models.
To investigate the relationship between the development of cancer and the subsequent occurrence of hard endpoints, such as death, reinfarction, and bleeding, the presence or absence of cancer was entered into a time-updated Cox model adjusted for the covariates, which were identified by the multivariable Cox model with backward elimination including the variables listed in Table 1; a P value<.1 in univariate analysis was the criteria used for inclusion in the final model. Cox models were stratified by study center and adjusted using the following covariates that were either statistically significant or plausibly related to mortality (age, sex, active smoker, diabetes, peripheral artery disease, human immunodeficiency virus (HIV) infection, type of ACS, Killip class, hemoglobin, creatinine, multivessel disease, complete revascularization, left ventricular ejection fraction, dual antiplatelet therapy, beta-blockers, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, statin), reinfarction (same covariates as for mortality in addition to prior coronary artery disease), and bleeding (same covariates as for mortality in addition to prior bleeding admission and in-hospital major bleeding according to BARC definition-type 3, 4 and 5).
Baseline characteristics of study population.
| Variables | |
|---|---|
| Age, y | 65.3±12.9 |
| Female sex, % | 25.4 |
| Body mass index, kg/m2 | 28.5±4.6 |
| Active smoking, % | 34.6 |
| Hypertension, % | 64.9 |
| Dyslipemia, % | 66.2 |
| Diabetes mellitus, % | 31.4 |
| Prior coronary artery disease, % | 28.8 |
| Peripheral artery disease, % | 7.8 |
| Prior heart failure, % | 3.9 |
| Prior stroke, % | 6.8 |
| Chronic obstructive pulmonary disease, % | 9.6 |
| Prior major bleeding, % | 2.2 |
| Human immunodeficiency virus infection, % | 0.3 |
| Hepatitis infection, % | 0.9 |
| Chronic corticosteroid therapy, % | 3.3 |
| ST-segment elevation myocardial infarction, % | 38.4 |
| Killip≥II, % | 15.3 |
| Left ventricular ejection fraction≤40%, % | 14.9 |
| Mitral regurgitation≥3, % | 4.5 |
| Severe aortic stenosis, % | 2.8 |
| Hemoglobin, g/dL | 14.1±1.8 |
| Leucocytes, count/μL | 9851.5±3760.2 |
| Creatinine, mg/dL | 1.0±0.6 |
| Atrial fibrillation at admission, % | 6.8 |
| Left bundle brunch block, % | 4.6 |
| Right bundle brunch block, % | 7.5 |
| Multivessel disease, % | 46.8 |
| Percutaneous coronary intervention, % | 73.8 |
| Drug-eluting stent, % | 59.0 |
| Coronary artery bypass graft, % | 5.4 |
| In-hospital reinfarction, % | 2.4 |
| In-hospital heart failure, % | 12.0 |
| In-hospital major bleeding, % | 2.0 |
| Clopidogrel, % | 61.6 |
| Ticagrelor, % | 16.4 |
| Prasugrel, % | 9.6 |
| Oral anticoagulation, % | 10.8 |
| Beta-blockers, % | 84.1 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, % | 76.1 |
| Aldosterone antagonist, % | 11.6 |
| Statin, % | 94.9 |
| Ezetimibe, % | 3.3 |
All P values<.05 were accepted as statistically significant. Statistical analysis was performed using R and STATA version 13.0 (Stata Corp LP.; Texas, United States).
ResultsBaseline characteristics and cancer incidenceBaseline characteristics of the study population can be consulted in Table 1. From the 3867 ACS patients, 182 were diagnosed of cancer during the follow-up (22.9±12.9months), with a cumulative incidence (Fig. 2A) of 2.47 per 100 patient/years (95%CI, 2.13–2.85). Mean time to cancer diagnosis in our population was 14.8±8.7months. The most common cancer site was skin, followed for lung, colon and rectum, urinary tract, and prostate (Fig. 2B).
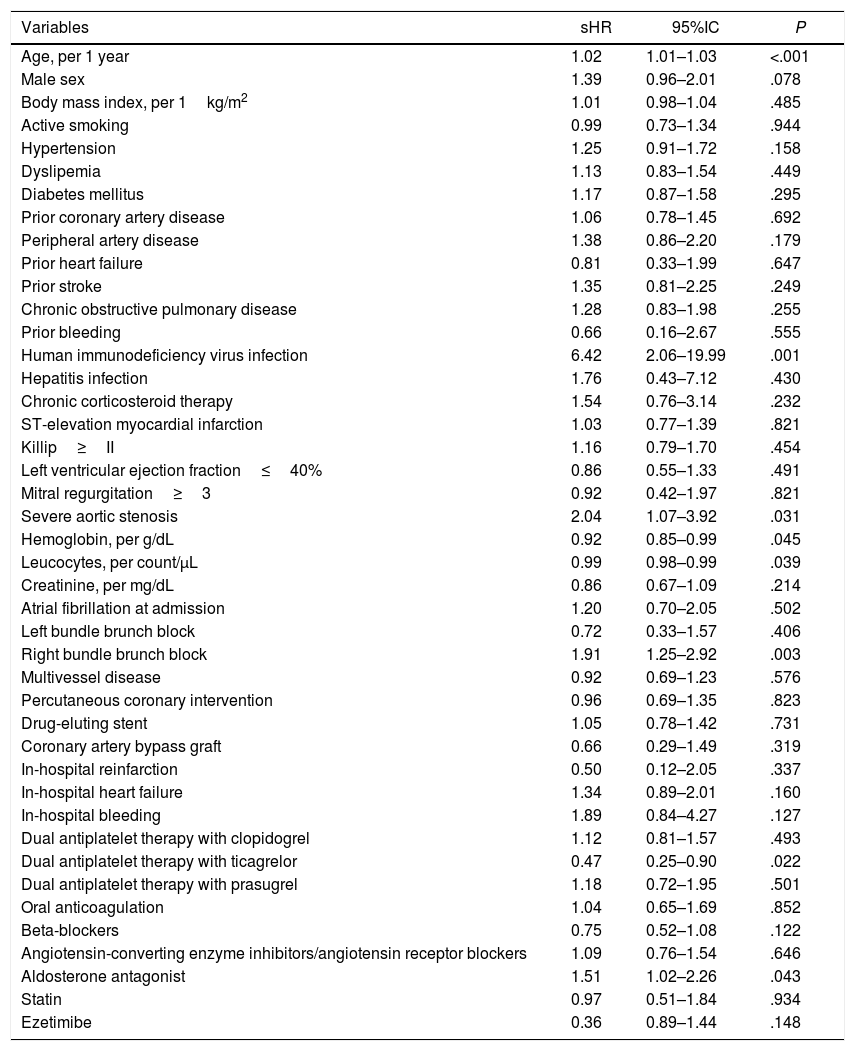
Predictors of cancer developmentThose clinical, analytical, echocardiographic, and hemodynamic variables associated with cancer development in the univariate analysis are shown in Table 2. Patients with post-ACS cancer were older, more likely to be male, to have severe aortic stenosis, anemia, and higher levels of leucocytes. Type of ACS, heart failure, and atrial fibrillation were not associated with the development of cancer. We have not found significant associations between medical therapy and cancer, except for aldosterone antagonists (higher risk of cancer) and ticagrelor (low risk of cancer).
Univariate analysis showing the association of clinical variables with development of post-discharge cancer.
| Variables | sHR | 95%IC | P |
|---|---|---|---|
| Age, per 1 year | 1.02 | 1.01–1.03 | <.001 |
| Male sex | 1.39 | 0.96–2.01 | .078 |
| Body mass index, per 1kg/m2 | 1.01 | 0.98–1.04 | .485 |
| Active smoking | 0.99 | 0.73–1.34 | .944 |
| Hypertension | 1.25 | 0.91–1.72 | .158 |
| Dyslipemia | 1.13 | 0.83–1.54 | .449 |
| Diabetes mellitus | 1.17 | 0.87–1.58 | .295 |
| Prior coronary artery disease | 1.06 | 0.78–1.45 | .692 |
| Peripheral artery disease | 1.38 | 0.86–2.20 | .179 |
| Prior heart failure | 0.81 | 0.33–1.99 | .647 |
| Prior stroke | 1.35 | 0.81–2.25 | .249 |
| Chronic obstructive pulmonary disease | 1.28 | 0.83–1.98 | .255 |
| Prior bleeding | 0.66 | 0.16–2.67 | .555 |
| Human immunodeficiency virus infection | 6.42 | 2.06–19.99 | .001 |
| Hepatitis infection | 1.76 | 0.43–7.12 | .430 |
| Chronic corticosteroid therapy | 1.54 | 0.76–3.14 | .232 |
| ST-elevation myocardial infarction | 1.03 | 0.77–1.39 | .821 |
| Killip≥II | 1.16 | 0.79–1.70 | .454 |
| Left ventricular ejection fraction≤40% | 0.86 | 0.55–1.33 | .491 |
| Mitral regurgitation≥3 | 0.92 | 0.42–1.97 | .821 |
| Severe aortic stenosis | 2.04 | 1.07–3.92 | .031 |
| Hemoglobin, per g/dL | 0.92 | 0.85–0.99 | .045 |
| Leucocytes, per count/μL | 0.99 | 0.98–0.99 | .039 |
| Creatinine, per mg/dL | 0.86 | 0.67–1.09 | .214 |
| Atrial fibrillation at admission | 1.20 | 0.70–2.05 | .502 |
| Left bundle brunch block | 0.72 | 0.33–1.57 | .406 |
| Right bundle brunch block | 1.91 | 1.25–2.92 | .003 |
| Multivessel disease | 0.92 | 0.69–1.23 | .576 |
| Percutaneous coronary intervention | 0.96 | 0.69–1.35 | .823 |
| Drug-eluting stent | 1.05 | 0.78–1.42 | .731 |
| Coronary artery bypass graft | 0.66 | 0.29–1.49 | .319 |
| In-hospital reinfarction | 0.50 | 0.12–2.05 | .337 |
| In-hospital heart failure | 1.34 | 0.89–2.01 | .160 |
| In-hospital bleeding | 1.89 | 0.84–4.27 | .127 |
| Dual antiplatelet therapy with clopidogrel | 1.12 | 0.81–1.57 | .493 |
| Dual antiplatelet therapy with ticagrelor | 0.47 | 0.25–0.90 | .022 |
| Dual antiplatelet therapy with prasugrel | 1.18 | 0.72–1.95 | .501 |
| Oral anticoagulation | 1.04 | 0.65–1.69 | .852 |
| Beta-blockers | 0.75 | 0.52–1.08 | .122 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 1.09 | 0.76–1.54 | .646 |
| Aldosterone antagonist | 1.51 | 1.02–2.26 | .043 |
| Statin | 0.97 | 0.51–1.84 | .934 |
| Ezetimibe | 0.36 | 0.89–1.44 | .148 |
95%CI, 95% confidence interval; sHR, subhazard ratio.
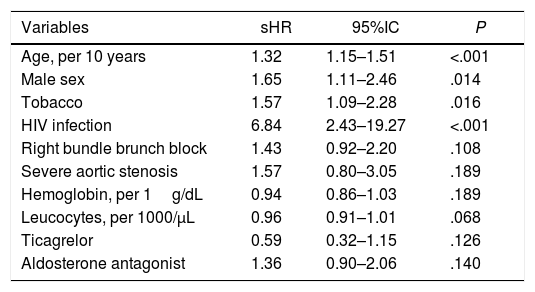
Table 3 shows the multivariate analysis. Age (sHR per 10 years, 1.32; 95%CI, 1.15–1.51; P<.001), male sex (sHR, 1.65; 95%CI, 1.11–2.46; P=.014), smoking (sHR, 1.57; 95%CI, 1.09–2.28; P=.016), and HIV infection (sHR, 6.84; 95%CI, 2.43–19.27; P<.001), resulted the only independent predictors of cancer development after ACS discharge. The c-index for final model was 0.68.
Multivariate analysis showing the independent predictors of post-discharge cancer.
| Variables | sHR | 95%IC | P |
|---|---|---|---|
| Age, per 10 years | 1.32 | 1.15–1.51 | <.001 |
| Male sex | 1.65 | 1.11–2.46 | .014 |
| Tobacco | 1.57 | 1.09–2.28 | .016 |
| HIV infection | 6.84 | 2.43–19.27 | <.001 |
| Right bundle brunch block | 1.43 | 0.92–2.20 | .108 |
| Severe aortic stenosis | 1.57 | 0.80–3.05 | .189 |
| Hemoglobin, per 1g/dL | 0.94 | 0.86–1.03 | .189 |
| Leucocytes, per 1000/μL | 0.96 | 0.91–1.01 | .068 |
| Ticagrelor | 0.59 | 0.32–1.15 | .126 |
| Aldosterone antagonist | 1.36 | 0.90–2.06 | .140 |
95%CI, 95% confidence interval; HIV, human immunodeficiency virus; sHR, subhazard ratio.
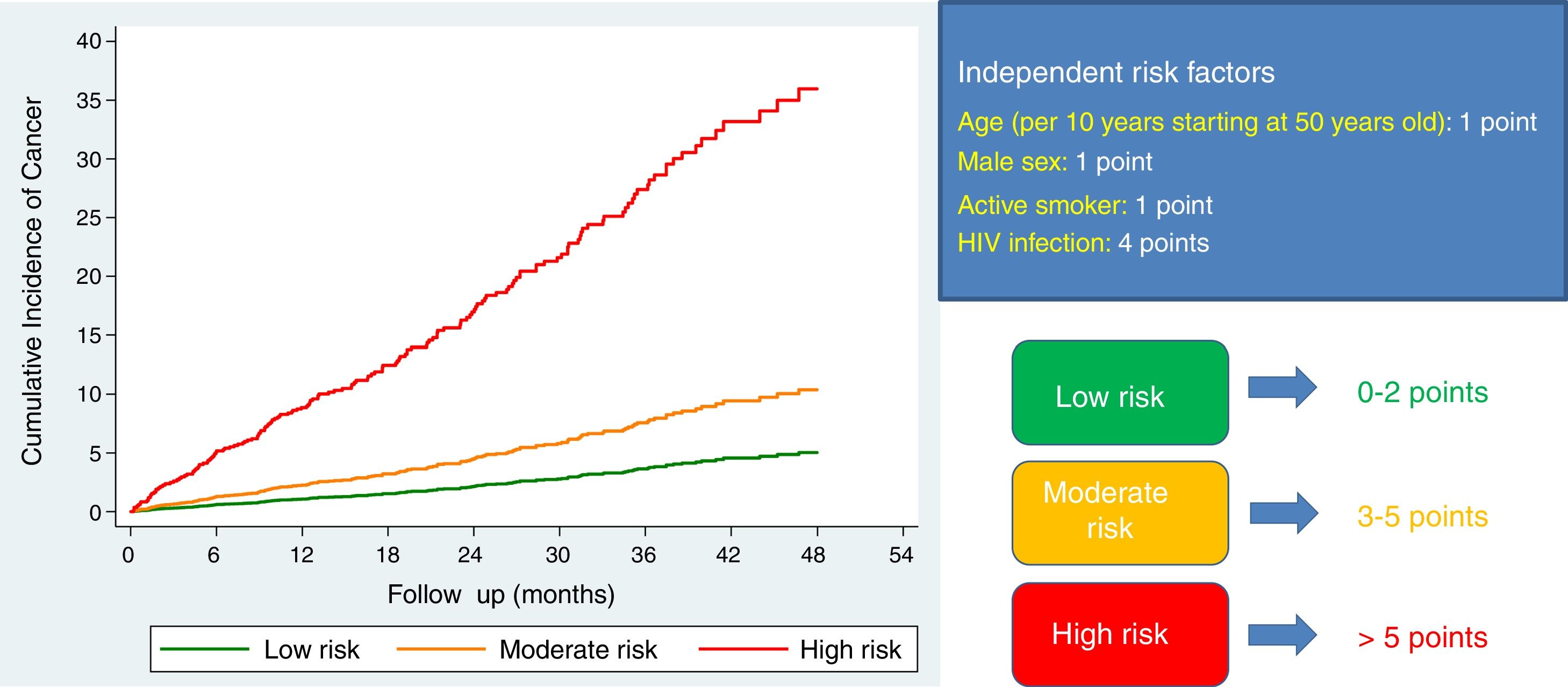
In order to make it easier for clinicians to estimate risk obtained from the multivariable model for predicting post-discharge cancer, we have constructed a simple risk score, based on the coefficients of these variables in the final model (1 point for each 10 years starting at 50 years old, 1 point for male sex, 1 point for active smokers, 4 points for HIV infection). Using this scoring system, we stratified our population according to the risk of developing cancer: low risk (0–2 points; n=1127), moderate risk (3–5 points; n=2683), and high risk (>5 points; n=57). The cumulative incidence of cancer steadily increased from 1.03 (95%CI, 0.71–1.50) per 100 patient/years at low risk group, to 2.35 (95%CI, 1.99–2.77) and 10.51 (95%CI, 5.82–18.98) per 100 patient/years at moderate and high risk groups, respectively (P<.001 for trend across strata; Fig. 3).
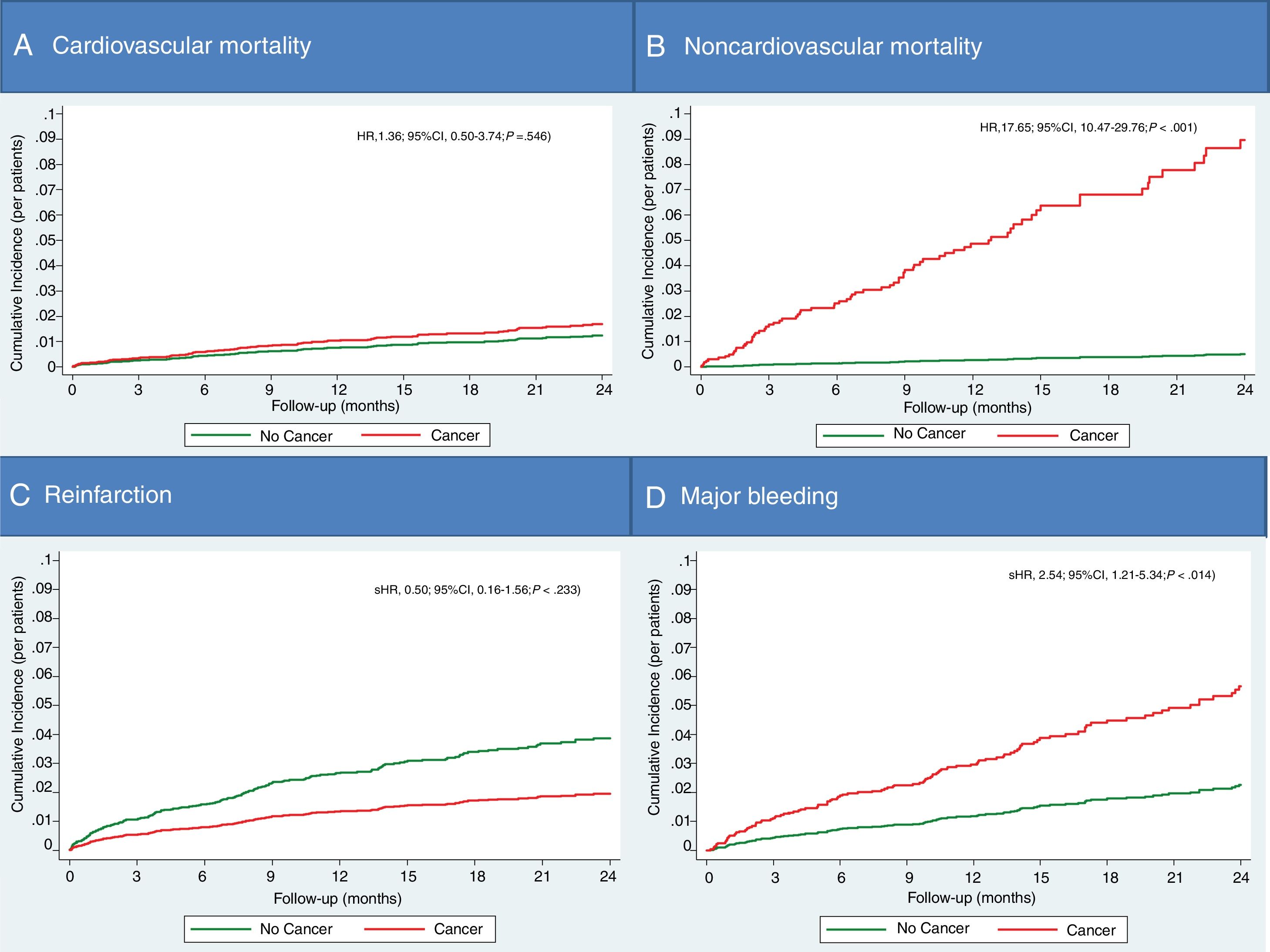
Cancer development and subsequent mortality, ischemic and bleeding eventsThe mortality of patients who have developed cancer after ACS discharge has occurred in 31 of the 821 cases (17%) during follow-up (7.7±9.9months), versus 246 of 3685 (6.7%) patients without post-discharge cancer (in 23.1±12.8months). De novo cancer after hospital discharge by ACS resulted in an independent predictor of follow-up mortality after adjusting for the other variables associated with mortality (HR, 3.77; 95%CI, 1.65–8.65; P=.002). The cardiovascular cause accounted for 55.3% of deaths in patients without cancer, versus only 12.9% of deaths in patients who developed post-discharge cancer. In this sense, post-discharge cancer has shown to be an independent predictor only for non-cardiovascular death (Fig. 4A,B).
There were no differences in the rate of reinfarction during the follow-up in patients with and without cancer (unadjusted sHR, 0.56; 95%CI, 0.18–1.74; P=.312) (Fig. 4C). However, major bleeding was more common in patients with cancer (unadjusted sHR, 2.93; 95%CI, 1.42–6.03; P=.003). After adjusting for potential confounding variables, post-discharge cancer remained as a predictor of bleeding (Fig. 4D).
DiscussionSummary of findingsThe present study analyzes the incidence, predictors, and prognosis of cancer development after hospital discharge by ACS. Using data of a retrospective contemporary registry from 2 tertiary hospitals, we have reported an annual incidence of post-discharge cancer of 2.47%. We have found only 4 independent predictors of cancer: age, male gender, active smoking, and HIV infection. Using a simple scoring system based on these 4 variables, we can stratify the risk of developing post-ACS cancer. Follow-up post-discharge cancer has proved to be an independent predictor of mortality and bleeding after an ACS, but not of reinfarction.
Interpretation of study findingsThe association between ACS and the future risk of cancer incidence is scarcely investigated. Few studies have investigated the association between ACS and the future risk of cancer. We have reported an annual cancer incidence of 2.47% after hospital discharge of ACS. This was similar to the recently published data from Tromsø Study in a large population of 28763 patients, with a crude annual incidence of cancer of 2.26% for patients with AMI versus 0.73% in patients without AMI.3 Interestingly, investigators from the Tromsø Study reported an increase in cancer incidence during the first 6 months after the AMI, with twice fold higher hazard ratio. Other 2 independent registries have suggested that AMI patients are at modestly increased risk of cancer, particularly of smoking-related cancers: Patients with ischemic syndromes in Stockholm country (n=63921) had an 8% higher risk of cancer,4 and similarly, a Danish registry-based cohort of 1 year survivors of AMI (n=96891) showed an overall 5% increased risk of cancer.5 Evidence of an association between atherosclerosis and neoplasm was also recently emphasized by a small cross-sectional study from Hong Kong showing a higher prevalence of advanced neoplasm in patients with proven coronary artery disease (>50% stenosis in any one of the major coronary arteries) than among patients whose angiograms did not show coronary artery disease.17
In our study, the most frequent type of cancer was skin cancer. This is consistent with the data from the National Cancer Institute,18 since non-melanoma skin cancer is the most frequent in the world. Possibly the data of our sample are due to that and not to risk factors shared specifically with the ACS.
The existence of a relation between AMI and cancer might help us to clarify the etiology of both diseases. It could also be relevant for the detection and monitoring of patients with these common and lethal diseases.19 Possible explanations for this association were proposed. It may be due to shared risk factors (eg, smoking, obesity, and low physical activity), a consequence of AMI diagnosis and treatment (eg, surveillance bias), or an AMI related (eg, chronic inflammation) impact on cancer risk.1 Growing evidence suggests that ACS and cancer share the same molecular pathways of disease development and that chronic inflammation plays a pivotal role in both carcinogenesis and atherosclerosis.2 However, the increased risk may merely be the result of diagnostic bias, as patients with AMI are hospitalized under close observation, increasing diagnostic testing. These diagnostic tests are also implemented more in patients under treatment with dual antiplatelet therapy after ASC since it favors bleeding and could make evident bleeding that was previously microscopic and unknown.
Also, occult cancer may induce a hypercoagulable paraneoplastic syndrome of which AMI is a clinical manifestation.20,21 In addition to this, occult bleeding from the tumor may cause anemia, precipitating AMI.22
Regarding the new-onset post-ACS cancer predictors, there is still less information. Age and smoking are independent predictors common to all studies.15 Age is the greatest risk factor for developing cancer. In fact, 60% of people who have cancer are 65 or older.23 Another important risk factor is smoking, which was widely associated with a marked increased risk of several types of cancer.24 In addition, male gender and HIV infection were the other independent cancer predictors in our study. People living with HIV remain at risk of AIDS-defining cancers in the era of effective antiretroviral therapy, and also have higher rates of several non-AIDS cancers than the general population, according to findings from a study of more than 86000 HIV-positive.25 Gender differential in cancer occurrence is a very consistent finding in descriptive epidemiology.26 For a very long time, it has been recognized that males are more prone to develop cancer. The analysis of age- and sex-specific cancer incidence data from Cancer Incidence in Five Continents provided by the International Agency for Research on Cancer documented the universal nature of the sex disparity in cancer.27 And the latest analysis of the National Cancer Institute Surveillance Epidemiology and End Results database showed the cancer incidence rate – for all sites combined – is 20% higher in men than in women.28
In our study, we have not found a significant association between heart failure or atrial fibrillation with the development of new-onset cancer. However, prior evidence-based in few studies has shown a possible association between heart failure and atrial fibrillation with cancer.29,30 Maybe the fact that we focus on a population of patients with ACS – with a low number of patients with heart failure and/or atrial fibrillation – can explain such inconsistencies.
It should also be remembered that drugs used in secondary prevention for AMI may influence the risk of cancer.6 In this sense, prolonged aspirin treatment has shown reduced mortality of several common cancers.7 However, studies on statin, angiotensin receptor blocker, and/or prasugrel treatment and future development of cancer are inconsistent in most cases.8–14 In our study, we have not found a significant association with cancer, neither for statins, nor for dual antiplatelet therapy, nor for angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, nor for beta-blockers. However, the main objection regarding the association of cardiovascular drugs to cancer in our research was the short study length. Moreover, in our study, most of the patients have been prescribed with aspirin and/or statins.
Finally, we have also studied the prognostic impact of post-discharge cancer in patients with ACS. As it is logical, patients who developed cancer had more non-cardiovascular mortality and more bleeding events. However, we have not found differences regarding the rate of reinfarction and cardiovascular mortality. Few studies have analyzed the prognosis impact of neoplasm detection during follow-up after ACS. In the recently published data from TRILOGY-ACS, authors found a higher rate of the combined end-point of cardiovascular death, AMI, and stroke, in patients with post-discharge cancer.8 Therefore, our results are consistent with data from TRILOGY-ACS study for the increased risk of death and bleeding events, but not for the risk of ischemic events.
Clinical implicationsOur study provides a representative and contemporary picture of the post-discharge cancer risk after ACS in patients seen in day-to-day practice. From the clinical viewpoint, the quantification of the risk of new-onset cancer at the time of hospital discharge is of value in that it provides the opportunity to enroll high-risk patients into proactive care management programs, to reduce costs from hospitalization for cancer while improving quality of care and patient functional status. Our simple model, based on 4 clinical variables, gives information early enough during the hospitalization – prior to discharge – to trigger a transitional care intervention, many of which involve discharge planning and begin before hospital discharge. This model could also be useful in the early detection of cancer after the identification of these risk factors in each patient. These measures of intensification of post-discharge care in patients at high risk of developing cancer would be aimed at reducing the death and bleeding rates.
LimitationsSome limitations should be acknowledged when interpreting our data. Results emanate from a retrospective study including ACS patients undergoing coronary angiography in two tertiary hospitals, with the limitations inherent to this type of design. The number of diagnoses of de novo cancer could be underestimated since the follow-up time is short and cancer requires a latency period. Although we have used a multivariable model to adjust for potential confounders, there may remain unmeasured or residual confounding variables. In this sense, a possibility to adjust for inflammatory markers would have strengthened the study. Moreover, due to a limited number of cancer events, we had limited statistical power to investigate the risk of specific cancer types. Regarding the risk score formulated, it is true that the most important predictor is HIV infection, a condition that is only present in a very low percentage of the study sample. This may expect to underestimate the risk in patients who do not have this disease.
ConclusionsThe annual incidence of new-onset cancer after ACS is low (2.5%). A score system based on 4 clinical factors (age, sex, smoking, and HIV infection) could help identify patients with higher risk of cancer. Development of post-discharge cancer resulted in an independent predictor of non-cardiovascular death and bleeding events, but it was not associated with an increased ischemic risk.
Cancer and ACS are 2 prevalent diseases with high morbidity and mortality. Currently, the relationship between both diseases is growing, both in shared risk factors and physiopathological factors that could be common. There are few studies on cancer-independent risk factors after ACS, but there are still fewer addressing the prognostic impact of cancer in this type of patient.
Does it contribute anything new?Our study indicates that the incidence of cancer after discharge from ACS is low, but there are identifiable risk factors from which we can perform a risk score. This score would be useful in early detection of cancer and its management during follow-up. We also observed that patients with ACS who develop cancer have a higher risk of non-cardiovascular mortality and bleeding.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Abbreviations: ACS, acute coronary syndrome; AMI, acute myocardial infarction.