The epidemiology of heart failure (HF) that complicates ST-segment elevation myocardial infarction (STEMI) is not well defined. Our aim was to analyze the epidemiology and prognosis of HF that complicates STEMI treated with primary percutaneous coronary intervention (PCI).
MethodsMulticentre registry of 14070 patients with STEMI treated with primary PCI from January 2010 to December 2015.
ResultsPatients with HF were older, more frequently female, and diabetic and had chronic ischaemic heart disease. Only 10.3% of patients with STEMI treated with primary PCI had HF on admission and the majority of them had the mildest form (Killip–Kimball II 77.8%). HF was associated with high mortality (30-day mortality was 2.9% of patients in Killip–Kimball I, 9.5% of Killip–Kimball II and 17.4% in Killip–Kimball III, P<.005, and 1-year mortality in patients who survived 30 days was 2.9%, 9.3% and 14.3%, P<.005, respectively). Both the presence of Killip–Kimball class II and III were independently associated with 30-day and 1-year mortality in 30-day survivors. Up to 6% and 15% of patients in Killip–Kimball II and III on admission worsened to cardiogenic shock, and the onset of cardiogenic shock during hospitalization was independently associated with mortality at 30-day and 1-year follow-up.
ConclusionsHF complicates 10.3% of patients with STEMI treated with primary PCI and it was associated with a higher risk of developing cardiogenic shock and with 30-day and 1-year mortality in 30-day survivors. In this high-risk population, primary PCI and posterior medical treatment should be prioritized to avoid worsening of HF.
La epidemiología de la insuficiencia cardiaca (IC) que complica el infarto de miocardio con elevación del segmento ST (IAMEST) no está bien definida. El objetivo fue analizar la epidemiología y la mortalidad de la IC que complica el IAMEST tratado con intervención coronaria percutánea (ICP) primaria.
MétodosRegistro multicéntrico de 14.070 pacientes con IAMEST tratados con ICP primaria desde enero de 2010 a diciembre de 2015.
ResultadosLos pacientes con IC fueron mayores, más frecuentemente mujeres y diabéticos y con mayor prevalencia de cardiopatía isquémica crónica. Solo el 10,3% de los pacientes con IAMEST tratados con ICP primaria tenían IC al ingreso, la mayoría la forma más leve (Killip-Kimball II 77,8%). La mortalidad a 30 días fue del 2,9% en Killip-Kimball I, del 9,5% en Killip-Kimball II y del 17,4% en Killip-Kimball III, p<0,005, y la mortalidad a un año en pacientes que sobrevivieron 30 días fue 2,9, 9,3 y 14,3%, p<0,005, respectivamente. La presencia de IC se asoció de forma independiente con la mortalidad a los 30 días y al año. Hasta un 6% y un 15% de los pacientes en Killip-Kimball II y III al ingreso empeoraron a shock cardiogénico, y su aparición durante la hospitalización se asoció de manera independiente con la mortalidad a los 30 días y al año.
ConclusionesLa IC está presente en el 10,3% de los pacientes con IAMEST tratados con ICP primaria, y se asoció con un mayor riesgo de desarrollar shock cardiogénico durante la hospitalización y con la mortalidad a 30 días y al año. En esta población de alto riesgo la ICP primaria y el tratamiento médico posterior se deben priorizar para evitar el empeoramiento de la IC.
Since the implementation of reperfusion therapy and, especially, primary percutaneous intervention (PCI), outcome of ST-segment elevation myocardial infarction (STEMI) has greatly improved.1 However, some clinical presentations are still associated with bad prognosis. Heart failure (HF) that complicates acute myocardial infarction (AMI) has long been recognized as a factor that increases in-hospital mortality.2 The latest European Society of Cardiology3 and ACC/AHA STEMI guidelines4 focused on cardiogenic shock, a clinical presentation associated with high mortality.5 However, little was mentioned in the guidelines about the epidemiology or prognosis of mild to moderate HF that complicates STEMI.2 This discrepancy between clinical impact and its relevance in the guidelines might be explained by the relatively scarce information available. Indeed, our knowledge of this complication is mainly based on studies that included all types of AMI (STEMI and non-STEMI) and reperfusion therapy in STEMI was underused.6–9 Therefore, the results of these studies might not be valid nowadays. The few studies carried out in STEMI patients treated with primary PCI were rather small or single centre,10–12 were based on patients included on randomized controlled trials13 or only evaluated short-term mortality.12,14 Although these studies showed that HF was associated with higher short-term mortality than patients without HF,11,13–15 1-year mortality has only been analyzed in one study.11 Moreover, little is known about the incidence of worsening HF during hospitalization in these patients.10,14 Hence, given the limitations of the studies analysing the prognosis of HF complicating STEMI not treated with state-of-the-art therapies, the aim of this study was to analyze the epidemiology and 30-day and 1-year prognosis of HF complicating AMI in a contemporary cohort of STEMI patients treated with primary PCI within a STEMI network.
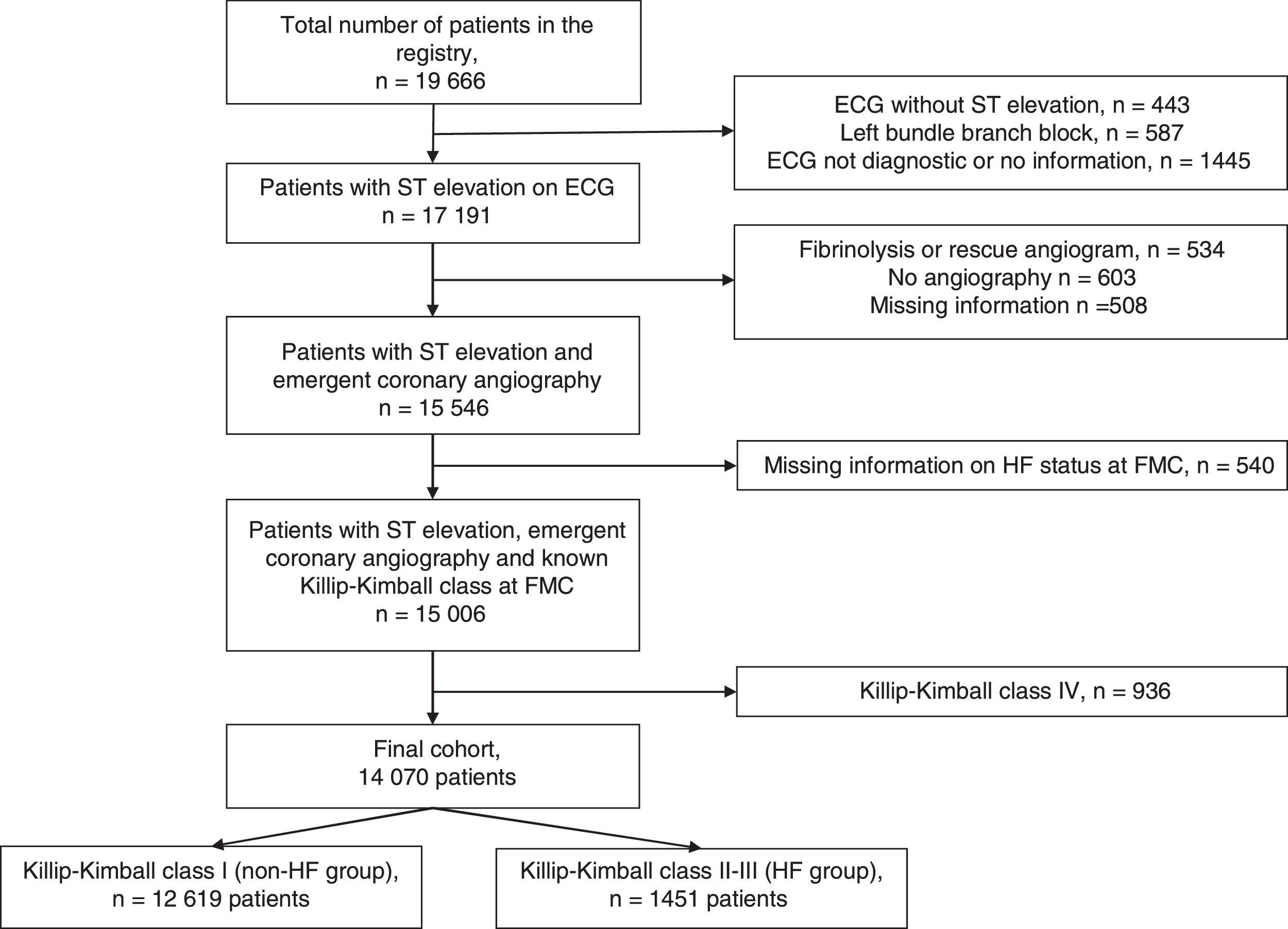
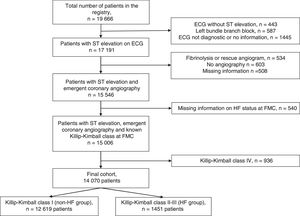
MethodsThe study was performed in the region of Catalonia (North-eastern Spain, population of 7553650 inhabitants in 2012) where a network for the treatment of STEMI (the ‘Codi IAM’ network) was officially launched in 2009. The set-up of the ‘Codi IAM’ network has been published elsewhere.16 Briefly, the network consisted initially of five centres with PCI facility open 24h/7 days a week and four daytime primary PCI centres that expanded progressively to a 8 centres with PCI facility opened 24/7 and 2 opened during office hours. The Emergency Medical System coordinates the logistics between the ambulances or community hospitals and the primary PCI hospitals. This network prioritizes primary PCI as the first-choice reperfusion treatment, provided it is carried out within 120min from first medical contact (FMC).3 The patient is transferred to the nearest primary PCI centre; and once treated and stable, the patient is transported back to the reference centre. All patients treated within the network are included in a prospective registry (Catalonia STEMI network registry) that belongs to the Health Department of the Catalonia Government and collects demographic, clinical and procedural data and acute phase mortality. The registry conforms to the ethical and legal requirements for research purposes in accordance with the ethical standards outlined in the Helsinki Declaration and was approved by the institutional ethics committee of each participating centre. The quality of the data included in the registry was verified by means of an external audit. Thirty-day and 1-year mortality analysis was based on official mortality registries from both the Catalan and Spanish governments. Thus, only patients living in Catalonia could be analyzed. Cause of dead was not recorded. Both the 30-day and 1-year mortality include in-hospital mortality. The presence of chronic ischaemic heart disease was defined as a history of previous myocardial infarction, PCI or coronary artery bypass grafting. Killip–Kimball class categorizes patients according to the physical examination. Patients in class I have no evidence of heart failure (HF). Patients in class II have findings consistent with mild to moderate HF, such as jugular venous distention or lung rales 1/2 way up the posterior lung fields or an S3. Patients in class III demonstrate overt pulmonary oedema, and patients in class IV were in cardiogenic shock.2 In-hospital onset of cardiogenic shock was registered since the start of the registry. Since 2012, information on in-hospital onset of acute pulmonary oedema was collected. From January 2010 to December 2015, 19666 consecutive cases were included in the Codi IAM registry. For the present analysis, only patients with chest pain and ST elevation on electrocardiogram at first medical contact (FMC) who underwent emergent coronary angiography were included. Exclusion criteria included patients with left bundle branch block, non-diagnostic electrocardiogram or electrocardiogram without ST elevation, treatment with fibrinolysis (with or without rescue angiography), patients with missing information on HF status on admission and patients in cardiogenic shock (Killip–Kimball class IV). Patients flow is depicted in Fig. 1. The final cohort included 14070 patients (71.5% of initial cohort). The primary end-point of the study was 30-day and 1-year mortality.
Continuous variables were summarized as mean and standard deviation, and categorical variables as proportions. Chi-square test for categorical variables and Student t test for continuous variables were used to assess the differences in baseline characteristics and outcome according to the presence of heart failure. P-value shows the comparison between the three groups (differences in >2 groups).
A logistic regression model was developed for short term (30-day) prognosis, adjusting for age, sex, diabetes, previous ischaemic heart disease, number of coronary artery with significant lesion, left main disease, haemorrhagic complications, arrhythmic complications, final diagnosis, angiography without PCI, primary PCI, total ischaemic time and in-hospital onset of cardiogenic shock. Simultaneous adjustment was chosen for all the variables included in the model through the Enter procedure. These variables were selected either because they presented statistically significant differences in the bivariate analysis or because they have been identified as potential confounding factors according to the literature. A Cox proportional hazard model was used for long-term prognosis (one-year mortality in 30-day survivors) with similar stratification and adjustment as for the 30-day endpoint. The analysis of proportionality assumption was carried out by means of log–log graphs, which showed parallel graphs for the different classes of the covariables. Kaplan–Meier survival curves were performed using patients without heart failure (Killip–Kimball I) as reference. We considered P-values <.05 from two-sided tests to indicate statistical significance. Analyses were performed with SPSS v24.
ResultsA total of 14070 patients were included, of whom 10% had HF at presentation. The majority of HF patients (78%) were on Killip–Kimball class II and only 22% presented with Killip–Kimball class III.
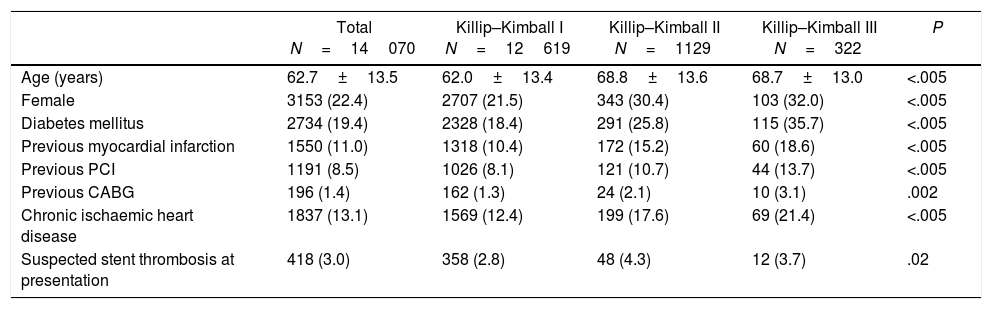
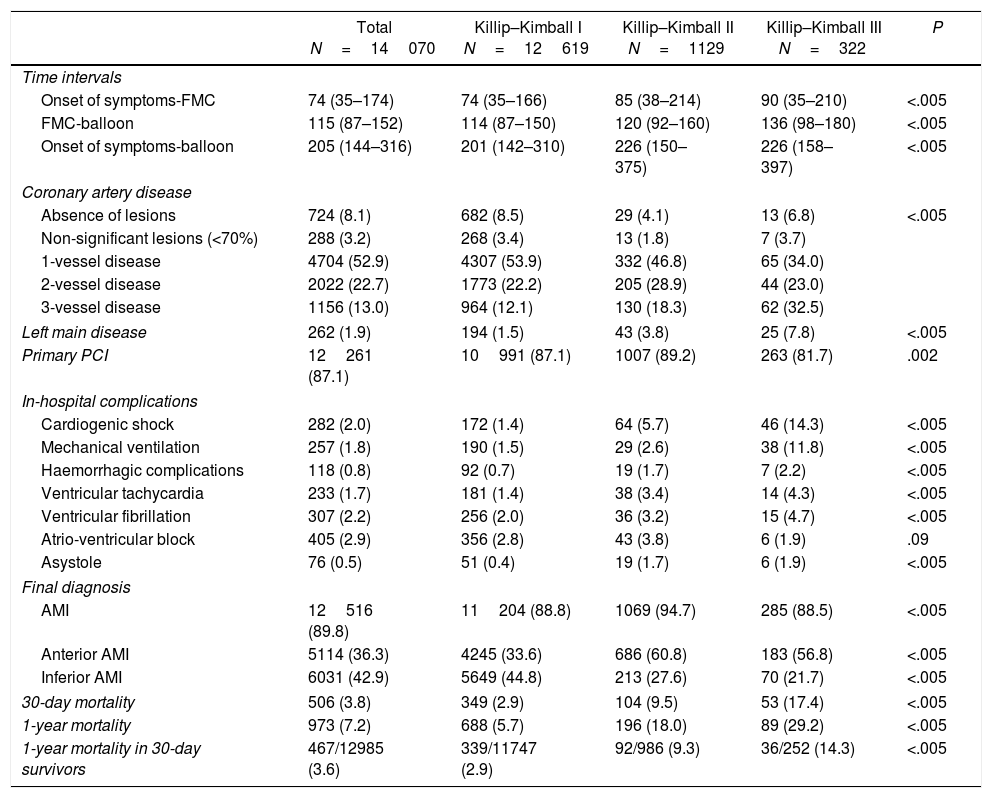
Baseline characteristics of patients with STEMI with and without HF at presentation are summarized in Table 1. Briefly, patients with HF were older, more frequently female and diabetic and had a higher prevalence of chronic ischaemic heart disease.
Baseline characteristics of patients with STEMI according to Killip–Kimball class at presentation.
| Total N=14070 | Killip–Kimball I N=12619 | Killip–Kimball II N=1129 | Killip–Kimball III N=322 | P | |
|---|---|---|---|---|---|
| Age (years) | 62.7±13.5 | 62.0±13.4 | 68.8±13.6 | 68.7±13.0 | <.005 |
| Female | 3153 (22.4) | 2707 (21.5) | 343 (30.4) | 103 (32.0) | <.005 |
| Diabetes mellitus | 2734 (19.4) | 2328 (18.4) | 291 (25.8) | 115 (35.7) | <.005 |
| Previous myocardial infarction | 1550 (11.0) | 1318 (10.4) | 172 (15.2) | 60 (18.6) | <.005 |
| Previous PCI | 1191 (8.5) | 1026 (8.1) | 121 (10.7) | 44 (13.7) | <.005 |
| Previous CABG | 196 (1.4) | 162 (1.3) | 24 (2.1) | 10 (3.1) | .002 |
| Chronic ischaemic heart disease | 1837 (13.1) | 1569 (12.4) | 199 (17.6) | 69 (21.4) | <.005 |
| Suspected stent thrombosis at presentation | 418 (3.0) | 358 (2.8) | 48 (4.3) | 12 (3.7) | .02 |
CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are mean±standard deviation or n (%).
All patients underwent emergent coronary angiography and 87.1% had a primary PCI (87.5% in the HF group and 87.1% in the no-HF group, P=.63), although primary PCI was done less frequently in patients in Killip–Kimball III (Table 2). The main reasons for not doing a PCI in the whole cohort were non-significant lesions in 356 cases, need for other treatment in 322 and medical decision in 262 patients. Time from onset of symptoms to first medical contact (FMC), FMC to balloon and total ischaemic time (onset of symptoms to balloon) were significantly longer in HF patients (all P<.05). The presence of 3-vessel disease, left main coronary artery disease and in-hospital arrhythmic (ventricular tachycardia and fibrillation, atrio-ventricular block or asystole) and haemorrhagic complications were more frequent in HF patients. Of note, 7.6% of patients with mild to moderate HF at presentation developed cardiogenic shock during the acute period compared to only 1.4% in non-HF patients, P<.005 (Table 2).
Time intervals, results of the coronary angiography, in-hospital complications, final diagnosis and in-hospital and 1 year mortality according to Killip–Kimball class on admission.
| Total N=14070 | Killip–Kimball I N=12619 | Killip–Kimball II N=1129 | Killip–Kimball III N=322 | P | |
|---|---|---|---|---|---|
| Time intervals | |||||
| Onset of symptoms-FMC | 74 (35–174) | 74 (35–166) | 85 (38–214) | 90 (35–210) | <.005 |
| FMC-balloon | 115 (87–152) | 114 (87–150) | 120 (92–160) | 136 (98–180) | <.005 |
| Onset of symptoms-balloon | 205 (144–316) | 201 (142–310) | 226 (150–375) | 226 (158–397) | <.005 |
| Coronary artery disease | |||||
| Absence of lesions | 724 (8.1) | 682 (8.5) | 29 (4.1) | 13 (6.8) | <.005 |
| Non-significant lesions (<70%) | 288 (3.2) | 268 (3.4) | 13 (1.8) | 7 (3.7) | |
| 1-vessel disease | 4704 (52.9) | 4307 (53.9) | 332 (46.8) | 65 (34.0) | |
| 2-vessel disease | 2022 (22.7) | 1773 (22.2) | 205 (28.9) | 44 (23.0) | |
| 3-vessel disease | 1156 (13.0) | 964 (12.1) | 130 (18.3) | 62 (32.5) | |
| Left main disease | 262 (1.9) | 194 (1.5) | 43 (3.8) | 25 (7.8) | <.005 |
| Primary PCI | 12261 (87.1) | 10991 (87.1) | 1007 (89.2) | 263 (81.7) | .002 |
| In-hospital complications | |||||
| Cardiogenic shock | 282 (2.0) | 172 (1.4) | 64 (5.7) | 46 (14.3) | <.005 |
| Mechanical ventilation | 257 (1.8) | 190 (1.5) | 29 (2.6) | 38 (11.8) | <.005 |
| Haemorrhagic complications | 118 (0.8) | 92 (0.7) | 19 (1.7) | 7 (2.2) | <.005 |
| Ventricular tachycardia | 233 (1.7) | 181 (1.4) | 38 (3.4) | 14 (4.3) | <.005 |
| Ventricular fibrillation | 307 (2.2) | 256 (2.0) | 36 (3.2) | 15 (4.7) | <.005 |
| Atrio-ventricular block | 405 (2.9) | 356 (2.8) | 43 (3.8) | 6 (1.9) | .09 |
| Asystole | 76 (0.5) | 51 (0.4) | 19 (1.7) | 6 (1.9) | <.005 |
| Final diagnosis | |||||
| AMI | 12516 (89.8) | 11204 (88.8) | 1069 (94.7) | 285 (88.5) | <.005 |
| Anterior AMI | 5114 (36.3) | 4245 (33.6) | 686 (60.8) | 183 (56.8) | <.005 |
| Inferior AMI | 6031 (42.9) | 5649 (44.8) | 213 (27.6) | 70 (21.7) | <.005 |
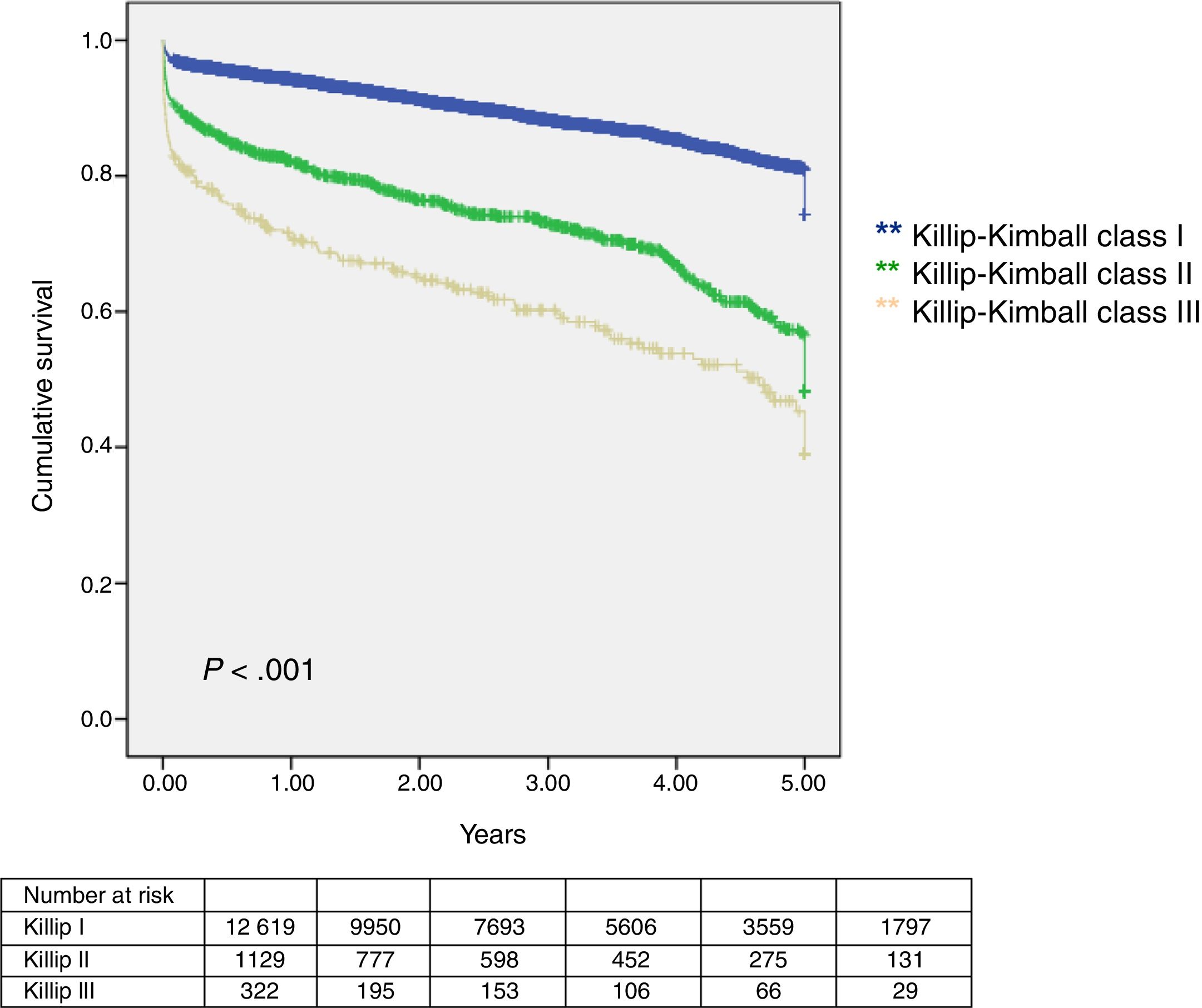
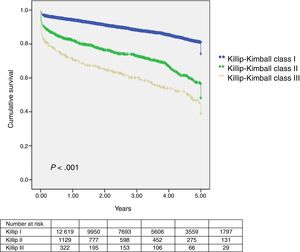
| 30-day mortality | 506 (3.8) | 349 (2.9) | 104 (9.5) | 53 (17.4) | <.005 |
| 1-year mortality | 973 (7.2) | 688 (5.7) | 196 (18.0) | 89 (29.2) | <.005 |
| 1-year mortality in 30-day survivors | 467/12985 (3.6) | 339/11747 (2.9) | 92/986 (9.3) | 36/252 (14.3) | <.005 |
AMI, acute myocardial infarction; FMC, first medical contact; PCI, percutaneous coronary intervention.
Data are mean±standard deviation, median (interquartile range) or n (%).
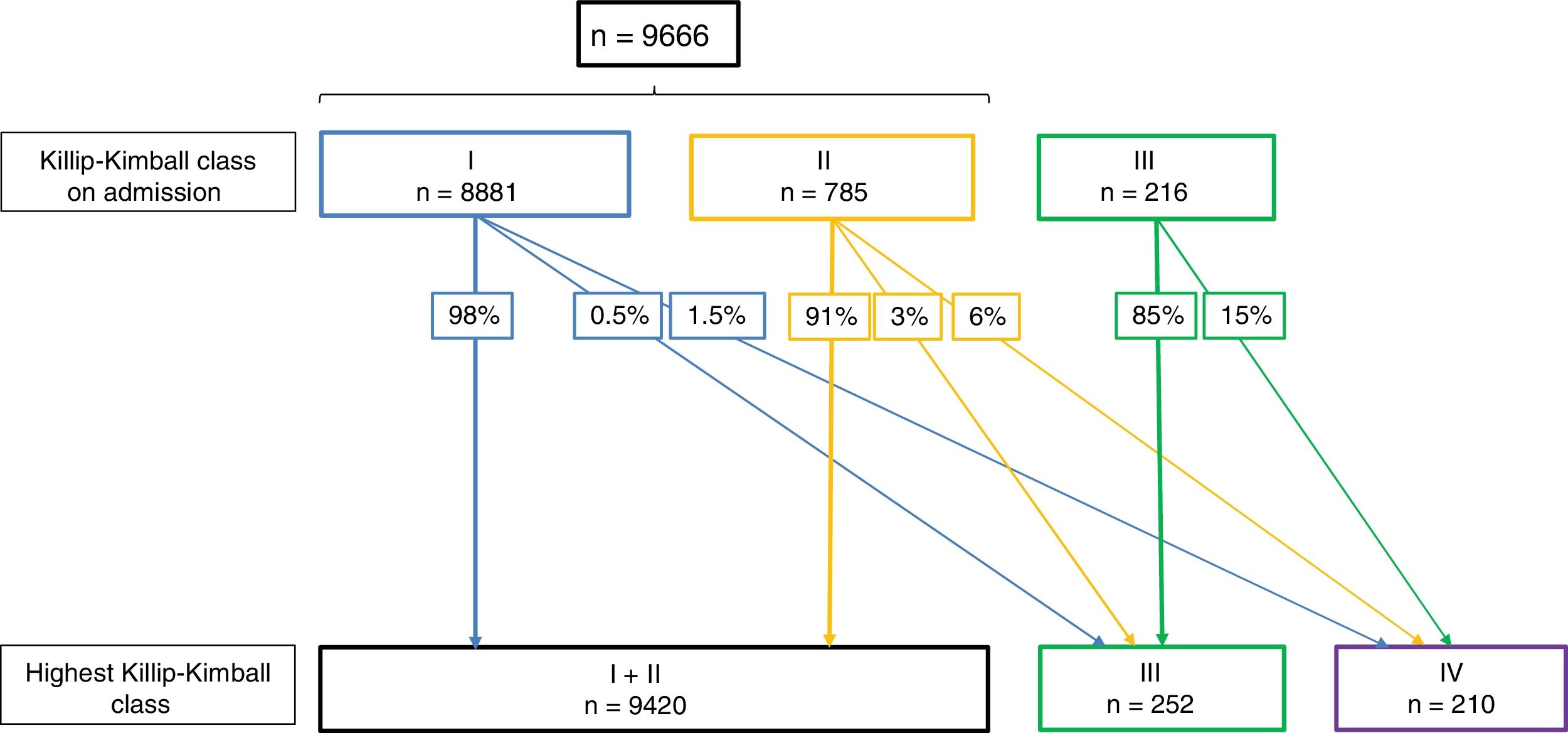
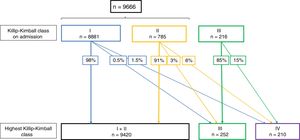
Fig. 2 shows the changes in Killip–Kimball class during hospitalization. As expected, the higher the Killip–Kimball on admission, the more likely it was that the patient worsened during hospitalization. Patients with STEMI and HF had more frequently a final diagnosis of AMI whereas other diagnosis (pericarditis, myopericarditis, Tako-Tsubo syndrome, pulmonary embolism and aortic dissection) were more frequent in non-HF patients (93.3% vs 88.8% and 5.9% vs 8.9%, respectively, all P<.005).
Changes in Killip–Kimball class during hospitalization. Since the presence of acute pulmonary oedema was not documented on 2010 and 2011, patients from 2010 and 2011 are excluded in this analysis. Killip–Kimball class 2 was not recorded as a complication, therefore Killip–Kimball ≤II are analyzed together.
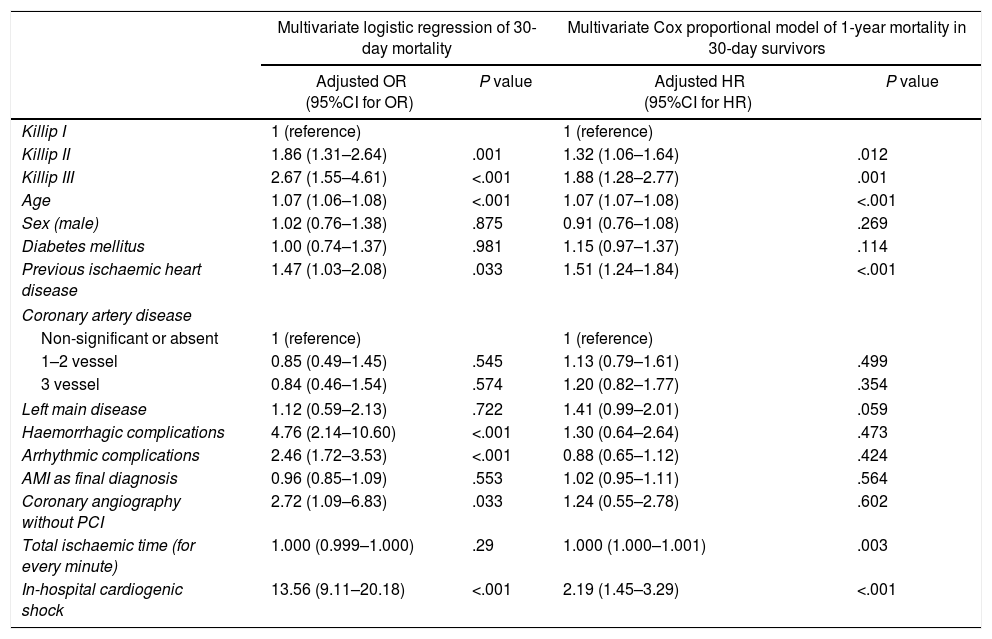
Median follow-up was 949 days (interquartile range 412–1520 days) and lost to follow-up was 4% (579 patients). Thirty-day and 1-year mortality was assessed in 13491 patients. Outcomes were worse in Killip–Kimball II and III compared to the absence of HF (Table 2 and Fig. 3). The presence of HF on admission was independently associated with a worse outcome at 30-day and 1-year in 30-day survivors (Table 3). Onset of cardiogenic shock during hospitalization carried the highest risk of mortality at both time points.
Multivariate logistic regression of 30-day mortality and multivariate Cox proportional model for the hazard ratio of 1-year mortality in 30-day survivors.
| Multivariate logistic regression of 30-day mortality | Multivariate Cox proportional model of 1-year mortality in 30-day survivors | |||
|---|---|---|---|---|
| Adjusted OR (95%CI for OR) | P value | Adjusted HR (95%CI for HR) | P value | |
| Killip I | 1 (reference) | 1 (reference) | ||
| Killip II | 1.86 (1.31–2.64) | .001 | 1.32 (1.06–1.64) | .012 |
| Killip III | 2.67 (1.55–4.61) | <.001 | 1.88 (1.28–2.77) | .001 |
| Age | 1.07 (1.06–1.08) | <.001 | 1.07 (1.07–1.08) | <.001 |
| Sex (male) | 1.02 (0.76–1.38) | .875 | 0.91 (0.76–1.08) | .269 |
| Diabetes mellitus | 1.00 (0.74–1.37) | .981 | 1.15 (0.97–1.37) | .114 |
| Previous ischaemic heart disease | 1.47 (1.03–2.08) | .033 | 1.51 (1.24–1.84) | <.001 |
| Coronary artery disease | ||||
| Non-significant or absent | 1 (reference) | 1 (reference) | ||
| 1–2 vessel | 0.85 (0.49–1.45) | .545 | 1.13 (0.79–1.61) | .499 |
| 3 vessel | 0.84 (0.46–1.54) | .574 | 1.20 (0.82–1.77) | .354 |
| Left main disease | 1.12 (0.59–2.13) | .722 | 1.41 (0.99–2.01) | .059 |
| Haemorrhagic complications | 4.76 (2.14–10.60) | <.001 | 1.30 (0.64–2.64) | .473 |
| Arrhythmic complications | 2.46 (1.72–3.53) | <.001 | 0.88 (0.65–1.12) | .424 |
| AMI as final diagnosis | 0.96 (0.85–1.09) | .553 | 1.02 (0.95–1.11) | .564 |
| Coronary angiography without PCI | 2.72 (1.09–6.83) | .033 | 1.24 (0.55–2.78) | .602 |
| Total ischaemic time (for every minute) | 1.000 (0.999–1.000) | .29 | 1.000 (1.000–1.001) | .003 |
| In-hospital cardiogenic shock | 13.56 (9.11–20.18) | <.001 | 2.19 (1.45–3.29) | <.001 |
95%CI, 95% confidence interval; AMI, acute myocardial infarction; HR, hazard ratio; OR, odds ratio; PCI, percutaneous coronary intervention.
This multicentre real-life registry is the biggest to date that has analyzed the prognostic role of mild to moderate HF complicating STEMI in patients treated with primary PCI. In this registry, only 10.3% of patients with STEMI treated with primary PCI had acute HF on admission and the majority of them had the mildest form (Killip–Kimball II in 77.8% of acute HF). The presence of HF in this setting carried a high mortality and was independently associated with 30-day and 1-year mortality in 30-day survivors. Up to 6% and 15% of patients in Killip–Kimball II and III on admission worsened to cardiogenic shock, respectively, and the onset of cardiogenic shock during hospitalization was independently associated with the highest mortality risk at 30-day and 1-year in 30-day survivors.
Around 7.3–35% of patients with AMI present with HF.1,10–14 In our study, only 10.3% of patients had HF at presentation (8% were in Killip–Kimball class II and 2.3% in Killip–Kimball class III). The broad difference in the incidence of HF on admission has different explanations. On the one hand, HF can be difficult to diagnose and classify in the acute setting, especially if it is not clearly patent.17 On the other hand, some studies included patients in cardiogenic shock on admission12,14 or a broad spectrum of AMI (including STEMI and non-STEMI).6–8 Finally, the incidence of HF that complicates AMI has decreased during the last decades.1,7,8 This can be explained by a higher use of revascularization therapies, a shorter time from symptom to primary PCI or fibrinolysis during the last 20 years and primary prevention medical strategies.1 Previous studies and our results show that time from symptom to admission or to PCI are longer in STEMI patients complicated with HF than in patients without HF.10,11,14,15 Indeed, we observed that patients with HF had longer time intervals: symptoms to first medical contact (FMC), FMC to balloon and total ischaemic time (symptoms to balloon). Patients with chest pain and heart failure may not identify their symptoms to be caused by an AMI and therefore they might seek medical advice later. Also, because these patients are more unstable at FMC, it may take a longer time to stabilize and carry them to the closest PCI centre.
Patients with HF were older, more frequently female and diabetic. These finding are consistent with previous studies.10–14 Similar to other studies, we saw a higher prevalence of previous chronic ischaemic heart disease.10,12
Although little can be done to prevent the onset of HF in STEMI before first medical contact, HF that occurs during hospitalization is likely associated with larger myocardial infarction and, therefore, interventions that decrease infarct size should be associated with a lower incidence of worsening HF during hospitalization. Studies carried out before the generalization of reperfusion therapies and that included all spectrum of HF have shown that the onset of HF after presentation at the hospital ranges from 9% to 42%.18–20 Given that reperfusion therapy is the gold standard for treatment of STEMI and that it has shown to decrease myocardial infarction size and improve survival in patients presenting with HF,21,22 it is expected that these numbers may decrease with contemporary treatment. Auffret et al. showed in a cohort of STEMI patients treated with primary PCI that HF was present in 11.8% of patients on admission and developed in-hospital HF in 9%,14 however no information was available on worsening HF in patients who had HF on admission. Santoro et al. showed that 1.3% of STEMI patients without HF on admission developed signs of HF during hospitalization.10 In our study, we saw that the higher the Killip–Kimball class on admission, the higher the likelihood of worsening HF during hospitalization. Thus, only 2% of patients with STEMI on Killip–Kimball class I at presentation complicated with pulmonary oedema or cardiogenic shock, whereas it happened in 9% and 15% of Killip–Kimball II and III, respectively. Tsai et al. showed similar results, with increasing development of hypotension/cardiogenic shock with higher Killip–Kimball class.11 Thirty-day mortality was strikingly high in patients with Killip–Kimball III (17.4%), whereas only 2.9% of patients in Killip–Kimball I died. Although the mildest grade of HF tends to be underestimated, 9.5% of Killip–Kimball II patients died at 30 days. Other studies have shown that mortality rates varies depending on the severity of HF (4.2–8% in Killip–Kimball class II and 9.8–27% in class III,11,13–15 23.6% in patients with HF (which included Killip–Kimball class II–IV).12 When patients in cardiogenic shock on admission or during hospitalization were excluded from the analysis, mortality has been reported to be as low as 1.1% in Killip–Kimball class I and 8% in patients with HF on admission.10 In multivariate analysis, we saw that Killip–Kimball class II and III were independent predictors of 30-day mortality, which is consistent with other studies.11,13,15 Interestingly, in-hospital acute haemorrhagic and arrhythmic complications had similar or higher risk of mortality than HF. Finally, in our study the factor that carried the highest mortality risk was the onset of cardiogenic shock during hospitalization, which could reflect a higher baseline risk and larger infarct size.
One-year mortality in patients with STEMI complicated with HF has rarely been analyzed. The two studies that analyzed 6-months survival in this group of patients found that mortality was significantly higher in patients with HF (HR, 1.92 and OR, 3.47).10,13 Tsai et al. showed that Killip III at presentation was independently associated with 1-year mortality (OR, 2.32, P=.012) and that the presence of advanced congestive HF was also independently associated with mortality (OR, 2.73, P=.003).11 Because 30-day mortality was high in patients with HF, we analyzed 1-year mortality in 30-day survivors. All-cause mortality was 2.9% in Killip–Kimball I, 9.3% in Killip–Kimball II and 14.3% in Killip–Kimball III. Both Killip–Kimball class II and III on admission were independently associated with 1-year mortality in 30-day survivors. Finally, the onset of cardiogenic shock during hospitalization was associated with the highest 1-year mortality risk.
LimitationsA limitation of our study is that information on the presence of chronic HF or left ventricular ejection fraction (LVEF) was not collected. The presence of chronic HF has been an exclusion criteria in a number of AMI studies.6,7,18 Few studies have reported the presence of chronic HF in patients with AMI with conflicting results. Whereas Wu el al. reported that history of HF was independently associated with lower in-hospital death,9 others reported that a history of chronic HF was associated with HF during hospitalization for an index AMI, which in turn was associated with higher mortality.6,8 The presence of LVEF <40–50% was more frequent in AMI patients presenting with HF patients.6,15,18 and lower LVEF was independently associated with higher in-hospital and 6 and 12 months mortality in patients with STEMI treated with primary PCI.11,13 However, it is noteworthy that, likely due to the generalization of reperfusion therapies, many patients with STEMI had LVEF >50%, even when they were in Killip–Kimball class III.11 Moreover, in AMI patients, the proportion of patients with HF and preserved LVEF (LVEF >50%) has increased. In contrast, those with HF and reduced LVEF have declined over time, and, irrespective of LVEF, patients with HF have high mortality.23 Finally, we excluded patients with left bundle branch block because in a study carried out in the same cohort, only 36% of patients with left bundle branch block had a coronary obstruction amenable of treatment with angioplasty.24
ConclusionsThis multicentre real-life registry of 14070 STEMI patients treated with primary PCI showed that 10.3% of patients had acute HF on admission. HF (both in Killip–Kimball class II and III) was associated with a higher risk of developing cardiogenic shock during hospitalization and with 30-day and 1-year mortality in 30-day survivors. In this high risk population, primary PCI and posterior medical treatment should be prioritized to avoid worsening of HF.
Heart failure is a complication of STEMI. However, most of the information available nowadays is based on studies that did not use primary percutaneous intervention.
Does it contribute anything new?This is a contemporary multicentre registry of 14070 non-selected patients with STEMI treated with state-of-the-art treatment. It offers information about 30-day and 1-year prognosis in patients with mild-to-moderate acute heart failure, which has not been widely studied in contemporary cohorts. The knowledge of the epidemiology and prognosis of acute heart failure in the setting of STEMI may allow us to identify high-risk patients and, thus, improve STEMI networks and resource allocation to the highest risk patients.
Funding was provided for the statistical analysis of the study by the Codi Infart program of the Pla director of malalties de l’aparell circulatori of the Health Department.
M. Grau was supported by the Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional-European Regions Development Fund FEDER (FIS CPII17/00012).
Conflict of interestNone declared.
The authors thank Dr. Carme Carré for statistical support and all participant centres of the Catalonian STEMI network (“Codi Infart”): Dr. Mónica Massotti (Hospital Clínic, Barcelona), Dr. Juan Francisco Muñoz (Hospital Mútua de Terrassa, Terrassa), Dr. Sergio Rojas (Hospital Joan XXIII, Tarragona), Dr. Xavier Carrillo (Hospital Germans Trias i Pujol, Badalona), Dr. Albert Ariza (Hospital de Bellvitge, Hospitalet de Llobregat), Dr. Mérida Cárdenas (Hospital Doctor Josep Trueta, Girona), Dr. Joan García-Picart (Hospital de Sant Pau, Barcelona), Dr. Rosa María Lidón (Hospital de la Vall d’Hebron, Barcelona), Dr. Carlos Tomás-Querol (Hospital Arnau de Vilanova, Lleida).