Antiplatelet agents such as acetylsalicylic acid (ASA) play a prominent role in preventing atherothrombosis. However, low-responsive patients who will not benefit from an increased dosage of this drug, which can cause bleeding and gastrointestinal irritation, need to be identified. Drugs such as omega-3 fatty acids, which enhance the vasodilating condition and diminish platelet aggregation, can potentiate the anti-aggregating effects of ASA, avoiding its side effects. Thus, we assessed the alternative use of 200mg/day of ASA and 100mg/day of this drug combined with 1g of omega-3 in 152 patients with chronic coronary artery disease.
MethodsOur analysis included platelet function (ASPItest), TBX2 concentrations (ELISA), and SNPs polymorphisms in the rs3842787 and rs3842798 regions of the PTGS1 gene of the COX-1 enzyme and the rs5918 region of the ITGB3 gene of the fibrinogen's receptor subunit glycoprotein IIIa.
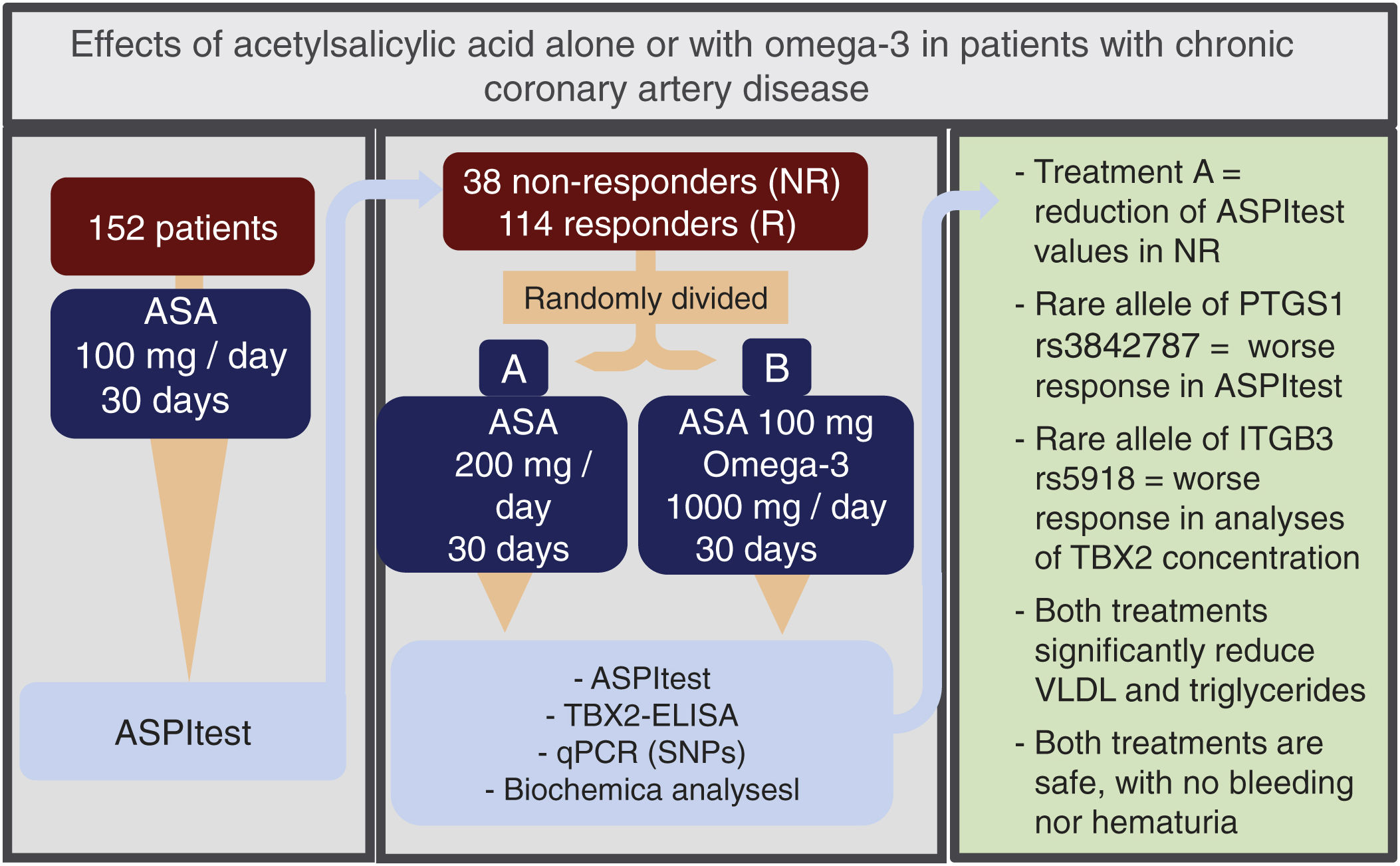
ResultsASPItest detected 38 non-responders. The reduction of ASPItest values was more significant in this group than in responders and fell to levels of responders in non-responders of the 200mg/day treatment. A rare allele of rs3842787 is associated with a worse ASPItest response, and the rare allele of the rs5918 polymorphism with a worse response related to TBX2 concentration. Both treatments showed no statistically significant difference in hematuria or bleeding, constituting safe treatment alternatives, and omega-3 treatment reduced monocyte levels.
ConclusionsOur results underscore the usefulness of pharmacogenetics for personalized treatments, avoiding gastrointestinal effects and undesirable bleeding.
El ácido acetilsalicílico (AAS) es ampliamente utilizado para prevenir la trombosis arterial. Sin embargo, deben identificarse pacientes refractarios para los cuales dosis altas de AAS son ineficaces y pueden causar irritación gastrointestinal y hemorragia. El fármaco omega-3 puede aumentar los efectos anticoagulantes del AAS y evitar sus efectos colaterales. Por ese motivo evaluamos el uso de 200mg/día de AAS o 100mg/día de AAS combinado con 1g de omega-3 en 152 pacientes con enfermedad coronaria crónica.
MétodosSe analizaron la función plaquetaria (ASPItest), las concentraciones de TBX2 (ELISA) y polimorfismos de SNP en las regiones rs3842787 y rs3842798 del gen PTGS1 de la enzima COX1y la región rs5918 del gen ITGB3 de la subunidad glicoproteína IIIa del receptor de fibrinógeno.
ResultadosEl ASPItest detectó 38 pacientes refractarios. La reducción de la función plaquetaria fue más significativa en este grupo que en los respondedores, cayendo a niveles de respondedores en los refractarios al tratamiento con 200mg/día. Un alelo raro de rs3842787 está asociado a una peor respuesta al ASPItest, y un alelo raro del polimorfismo rs5918, a una peor respuesta en el análisis de la concentración de TBX2. Los tratamientos analizados no mostraron evidencia estadísticamente significativa de hematuria o de sangrado, y el tratamiento con omega-3 mostró reducción en los niveles de monocitos.
ConclusionesLa farmacogenética es una herramienta indispensable para una medicina personalizada con tratamientos que eviten efectos gastrointestinales y hemorragias, como los descritos en este estudio.
The primary pathophysiological mechanism of atherosclerosis is the chronic inflammation caused by the deposit of lipids, which promotes the formation of occlusive thrombi.1 The clinical consequence of chronic arterial thrombosis is chronic coronary artery disease.2 Arterial thrombotic events associated with atherosclerosis include the formation of atheromatous plaques.3 In this scenario, platelets play a fundamental role in the thrombotic process, providing the initial hemostasis of vascular injury and precipitating thrombus formation in patients with atherosclerotic disease.4
Platelet stimulation releases arachidonic acid, which, under the action of the cyclooxygenase (COX) enzyme, is transformed into the prostaglandin thromboxane A2. Thromboxane A2 stimulates platelet activation and the release of adenosine diphosphate, which induces irreversible platelet aggregation through P2Y12 receptors.5 Acetylsalicylic acid (ASA) acetylates serine from position 529 of the COX-1 polypeptide chain, irreversibly inhibiting the effects of this enzyme and the formation of thromboxane A2. In addition, ASA has anticoagulant, vascular, anti-inflammatory, and pro-fibrinolytic effects.6
Thus, antiplatelet agents like ASA have a central function in the primary and secondary prevention of atherothrombosis.7 However, despite its proven benefit, some patients using ASA continue to suffer atherothrombotic events,8 and approximately 20% will have some recurrent vascular event in a period of five years.9 For this reason, it is necessary to identify patients eventually showing low response to treatment.10 In addition, some crucial adverse side effects, such as bleeding, remain in debate and raise the possibility of creating a specific therapy for each patient.4
Specific therapies do not necessarily refer to the increase in the dosage of ASA, nor does it imply a reduction in the number of low-responsive patients, as demonstrated by data from the Antiplatelet Trialists’ Collaboration.11 These data reveal that doses of 160–325 and 500–1000mg are equally effective as treatments with 75–150 and 160–325mg/day. In addition, that study showed that the major limitation to the increase in dosage is related to gastrointestinal bleeding and irritation. Therefore, adding other drugs, such as omega-3 fatty acids, can potentiate the antiaggregant effects of ASA without its inconvenient side effects.12 Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid reduce arachidonic acid cellular levels by competing with this compound in several metabolic pathways, inhibiting its synthesis from linoleic acid, and interfering in the interaction with the 2-acyl position in phospholipid membranes.13 Eicosapentaenoic acid competes with arachidonic acid as a substrate for the COX enzyme, inhibiting the production of thromboxane A2 by platelets. In endothelial cells, prostaglandin 2 production is weakly inhibited, with eicosapentaenoic acid-produced prostaglandin 3 acting additively to prostaglandin 2.14 This inhibition results in a favorable deviation of the hemostatic balance to an augmented vasodilating condition with diminished platelet aggregation.15
The refractory effect in ASA non-responsive individuals is due to several factors, among which the single-nucleotide polymorphisms (SNPs) stand out.16 Thus, pharmacogenetics can effectively contribute to identifying and treating ASA-responsive and low-responsive patients.17 In low-responsive individuals, ASA's generally used dose of 100mg/day may be insufficient for inhibiting platelet aggregation.17 Dose adjustments up to 200mg/day may reduce the number of non-responders. The need for this adjustment may be related to different risk factors for atherosclerotic disease.17 In addition, treatment with 100mg/day of ASA and 1000mg/day of omega-3 fatty acid can reduce the number of non-responders to classical treatment and avoid undesirable effects such as bleeding and gastrointestinal alterations.17
Thus, the objective of this study was to evaluate the use of 200mg/day of ASA or 100mg/day of ASA combined with 1g of omega-3 fatty acid in the response of patients with chronic coronary artery disease refractory to previous use of 100mg/day of ASA and to determine polymorphisms of SNPs associated with treatment resistance. For this purpose, pharmacological analyses were performed, including in vitro platelet aggregation studies and genetic polymorphism analyses.
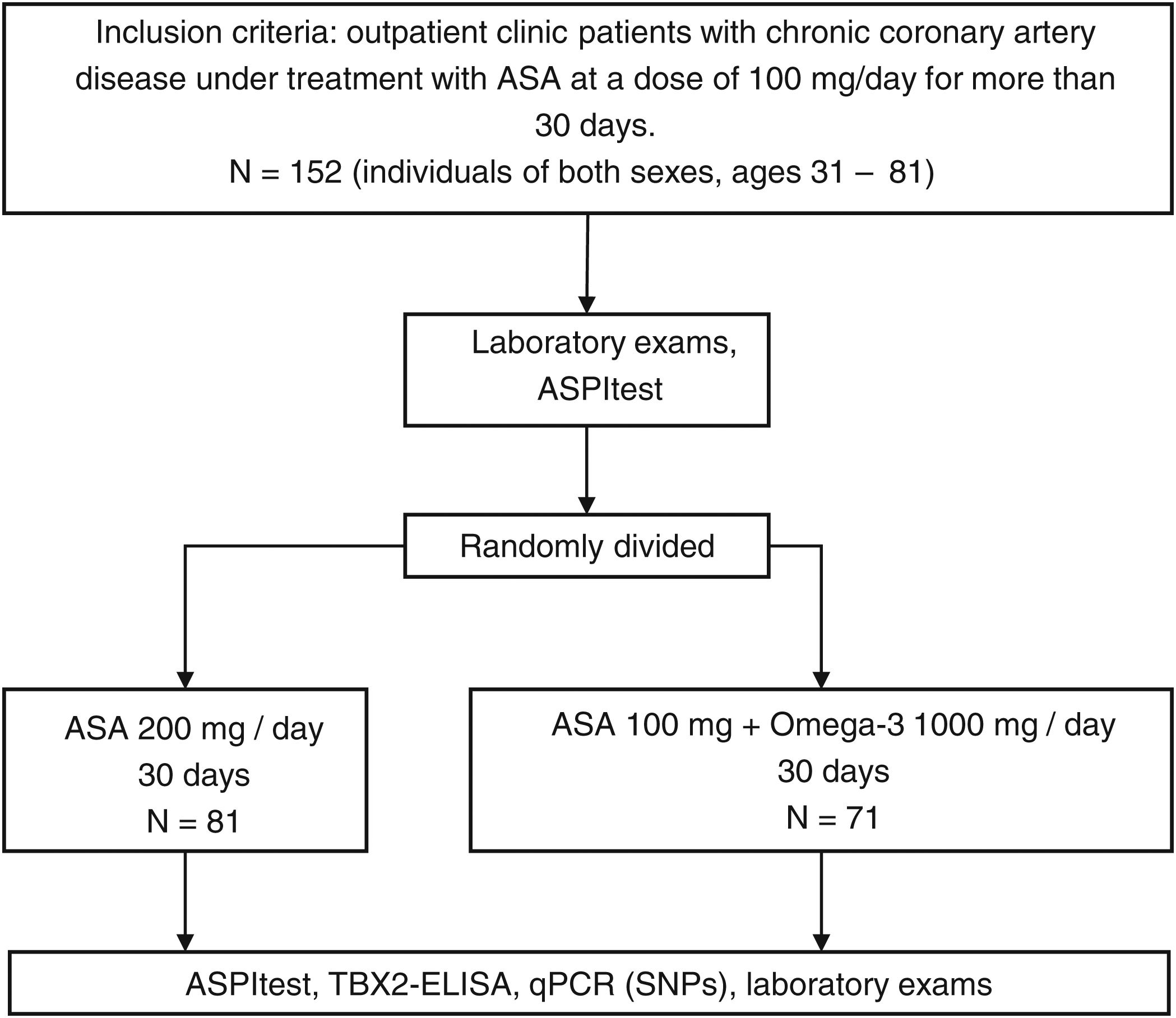
MethodsThis is an analytical, experimental, and prospective study conducted between May 2013 and October 2015 in 152 patients screened at the outpatient clinic of the Dante Pazzanese Cardiology Institute (Fig. 1). This sample included individuals of both sexes, ages 31–81, with chronic coronary artery disease and under treatment with ASA at a dose of 100mg/day for more than 30 days (Fig. 2). All participants signed an Informed Consent Form previously approved by the Research Ethics Committee of the Dante Pazzanese Cardiology Institute. The same committee approved this study under registration number CAAE:22534113.0.0000.5462. The authors are responsible for the fidelity of the study, the accuracy of the analyses, and data completeness.
Central illustration. Trial design and main outcomes showing the effectiveness of the treatment and the usefulness of the pharmacogenetic approach to choose the best treatment for non-responders patients. ASA: acetylsalicylic acid; NR: non-responders; R: responders; PCR: polymerase chain reaction; VLDL: very low-density lipoprotein; SNP: single-nucleotide polymorphism; qPCR: quantitative real-time polymerase chain reaction.
In a first clinical evaluation (Time A) of participants under 10–12h of fasting and 24h after ingestion of the 100mg/day dose of ASA (group ASA100), samples were collected for laboratory tests. Subsequently, patients were randomly divided into two treatment groups showing clinical and demographic data distributed homogeneously and without statistically significant differences (Table 1 of the supplementary data). Both groups underwent 30 days of treatment with the adjusted dosage. The ASA200 group received a dosage of 200mg/day of ASA, and the ASA100O3 group received l00mg of ASA and 1g of omega-3 fatty acid per day. At the end of the 30-day treatment and 24h after administration of the last dose of the corresponding drugs, the second evaluation (Time B) of the patients was performed. In this clinical evaluation, samples were also taken for a new round of laboratory analyses.
Biological samples and laboratory analysesBlood collection for biochemical and DNA analyses included the use of four 10ml dry tubes, four 10ml tubes with ethylenediaminetetraacetic acid, two 10ml tubes containing citrate, and one 10ml tube containing fluoride. In addition, one 0.5ml tube containing hirudin was used for blood collection from each patient for platelet aggregation analysis on the Multiplate platform (Roche, Switzerland). A 5ml vial containing the first-morning urine, or from minimum retention of four hours, was also collected for urinalysis. Serum and nucleic acid voucher samples were stored in the Molecular Biology Laboratory for further studies after approval by the Ethics and Research Committee and with the patient's authorization.
Biochemical tests using enzymatic–colorimetric and immunochemical methods were performed at the Clinical Laboratory of the Dante Pazzanese Institute. Thus, glycemia, total cholesterol as well as high-density lipoprotein (HDL), low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) fractions, triacylglycerides, complete blood count, serum urea, serum creatinine, serum uric acid, aspartate transaminase, alanine transaminase, fibrin degradation products (D Dimer), high-sensitive C-reactive protein and Urine I (Ta 1) were determined.
The platelet function of the samples from the ASA100, ASA200, and ASA100O3 groups was determined using the Multiplate analyzer platform (Roche, Switzerland). Analysis of cyclooxygenase-1 (COX-1) inhibition by ASA was performed using ASPItest (Roche, Switzerland), a reagent containing the COX-1-specific agonist arachidonic acid. Platelet function was also assessed using TRAPtest (Roche, Switzerland), which contains the thrombin's receptor activator peptide-6 (TRAP-6), a potent platelet activator. These analyses were performed according to the manufacturer's instructions. The results of the ASPItest at Time A were used to classify patients into ASA responders and non-responders (Table 2 of the supplementary data). Thus, patients were considered “highly responsive” up to 30 aggregation units (AU), “responders” from 30 to 40AU, and “non-responders” above 40AU.
Plasma determination of thromboxane B2Dosage level of thromboxane B2 (TBX2) was performed on plasma samples from whole peripheral blood collected in an ethylenediaminetetraacetic acid-containing tube. TBX2 concentrations were determined using the Cusabio Human Thromboxane B2 ELISA kit according to the manufacturer's instructions. The quantification of TBX2 was performed by spectrophotometry, by reading absorbance at a wavelength of 450nm, using the Multiskan GO platform (Thermo Fisher Scientific, United States). The readings were performed in duplicate and monitored using the standard curve provided by the manufacturer.
Analysis of DNA polymorphismsThe SNPs polymorphisms for the C/T substitution in the rs3842787 and rs3842798 regions of the PTGS1 gene of the COX-1 enzyme and the SNP for the T/C substitution in the rs5918 region of the ITGB3 gene of the glycoprotein IIIa (GPIIIa) subunit of the fibrinogen receptor were analyzed. These SNPs were chosen based on the information available in the NCBI's dbSNP database.18
Genomic DNA for these analyses was extracted from whole peripheral blood using the QIAamp DNA Blood Max Kit (QIAGEN, GmbH, Germany) and the QIAcube automated system (QIAGEN, GmbH, Germany). From these samples, the polymorphic regions were amplified by polymerase chain reaction (PCR) using the primer pairs 5′-CAGAGGAAGTAAGCGGCAG-3′/5′-CCCCATCCCACCAAAAGACT-3 (PTGS1 rs3842787 region of 497bp), 5′-GGGGATAGGAGTGGAAATAGGG-3′/5′-CTATGAGGATGAGGCGGTC-3′ (PTGS1 rs3842798 region of 496bp), and 5′-TGCTCCAATGTACGGGTAA-3′/5′-TCCCCAAGACTTCCTCCTCA- (ITGB3 rs5918 region of 396bp). The three primer pairs were selected using the Primer3 program v.0.4.019 and sequences available in the NCBI18 and Ensembl20 databases. The amplification reactions of the genomic DNA extraction samples were prepared using 1U of Taq DNA polymerase (DNA Express Biotechnology, Brazil), 1× PCR buffer (DNA Express Biotechnology, Brazil), 200μM of each dNTP (GE Healthcare, England), and 100nM of each primer (DNA Express Biotechnology, Brazil), and conducted in a Veriti thermocycler (Applied Biosystems, United States). The PCR conditions consisted of an initial denaturation at 95°C for 3min, followed by 40 cycles of denaturation at 95°C for 30s, hybridization at 62°C for 30s, and extension at 72°C for 1min, ending with an extension at 72°C for 10min.
PCR products were analyzed by horizontal electrophoresis in a 1% agarose gel immersed in 0.5× TBE buffer (90mM Tris–HCl, 90mM boric acid, 2.0mM EDTA pH 8.0) for 30min at 100V and 60mA. DNA was visualized under ultraviolet light after incorporating the intercalating agent GelRed (Biotium Inc., Hayward, United States). PCR products that showed fragments of expected size with an absence of nonspecific amplification were purified using the Illustra GFX PCR DNA and Gel Band kit (GE Healthcare, England). After treatment with the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems), these purifications were sent to the Human Genome Study Center (Instituto de Biosiências, USP, Brazil) for sequencing on an ABI 3730 DNA Analyzer (Life Technologies – Applied Biosystems). Sequencing results were analyzed in the Sequencing Analysis 5.3.1 software, using Base Caller KB and BioEdit Sequence Alignment Editor software. Some of these samples from the 3 genotypes confirmed by Sanger sequencing were used as controls in real-time PCR (qPCR) reactions.
The qPCR analyses were performed on a RotorGene thermocycler (Qiagen GmbH, Hilden, Germany) using the TaqMan SNP Genotyping Assay kit (Life Technologies, United States) and two probes for each polymorphism. These two probes, homologous to each of the studied alleles, are marked with 6-carboxyfluorescein (FAM probe) and 6-carboxy-4,7, 2′, 7′-tetrachlorofluorescein (VIC probe). VIC/FAM labeling was used to identify C/T substitution polymorphism in PTGS1 rs3842787 and PTGS1 rs3842798 genes, and FAM/VIC labeling for T/C substitution polymorphism in ITGB3 rs5918 gene. The amplification reactions of the genomic DNA samples were prepared using reagents from the TaqMan Genotyping MasterMix Kit (Life Technologies, United States) according to the manufacturer's indications, 0.5μl of Taqman SNP Genotyping Assay 40× (Life Technologies, United States), and DNase-free water to a final volume of 20μl. The qPCR conditions consisted of a 10min cycle at 95°C for activation of the HotStar Taq DNA polymerase, followed by 50 cycles of 15s at 95°C for DNA denaturation and ending with 90s at 60°C for probe hybridization and DNA extension. The data were analyzed using the Rotor-Gene Q – Pure detection program (QIAGEN, GmbH, Germany). At the end of the reactions, approximately 10% of the samples were sequenced by the Sanger method for genotyping confirmation.
Statistical analysisBefore comparing the quantitative variables of the various analyses, the Kolmogorov–Smirnov test was used to assess whether the parameters followed a normal distribution. The parametric Student's t-test was used for data with normal distribution. For the remaining data, the non-parametric Mann–Whitney or Wilcoxon tests were used. To compare the frequencies of the qualitative variables, the Chi-square test was performed. All statistical analyses were conducted using the SPSS 20 software (SPSS Inc., United States), and the significance level adopted was P<.05. Still, the Hardy–Weinberg equilibrium was calculated for the study population after genotyping the polymorphisms in the PTGS1 and ITGB3 genes.
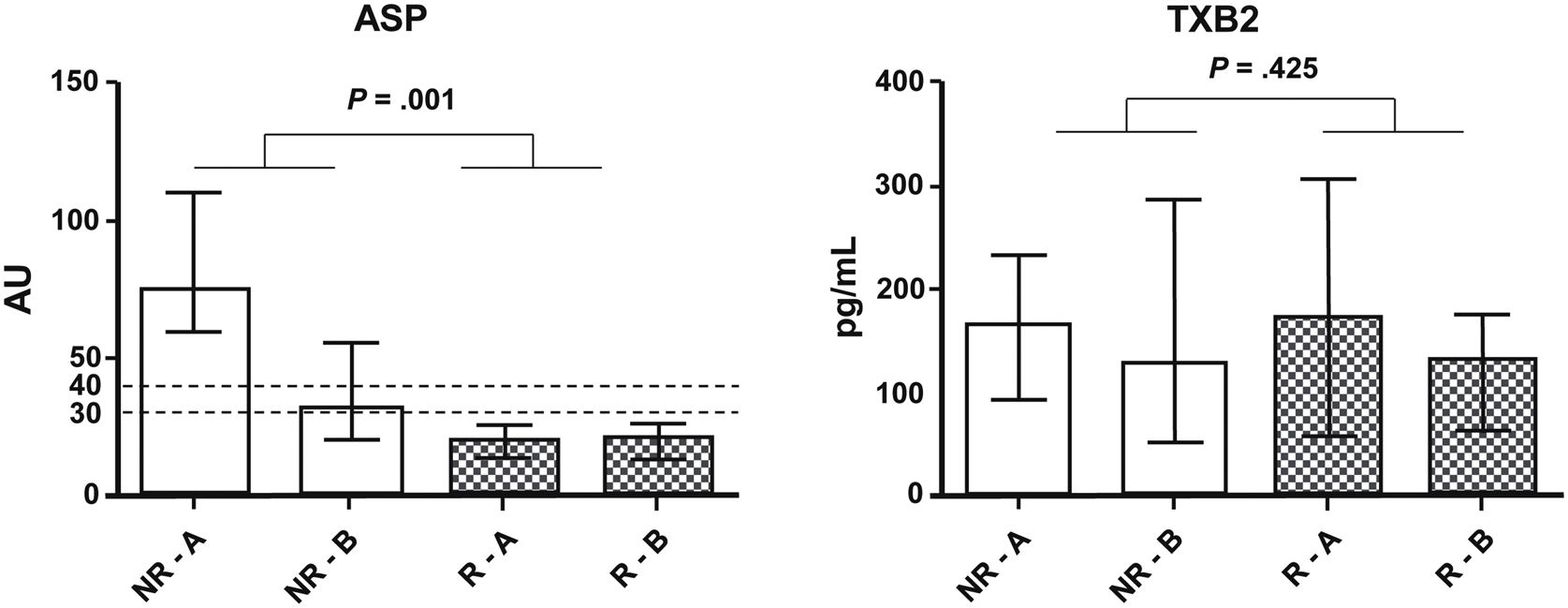
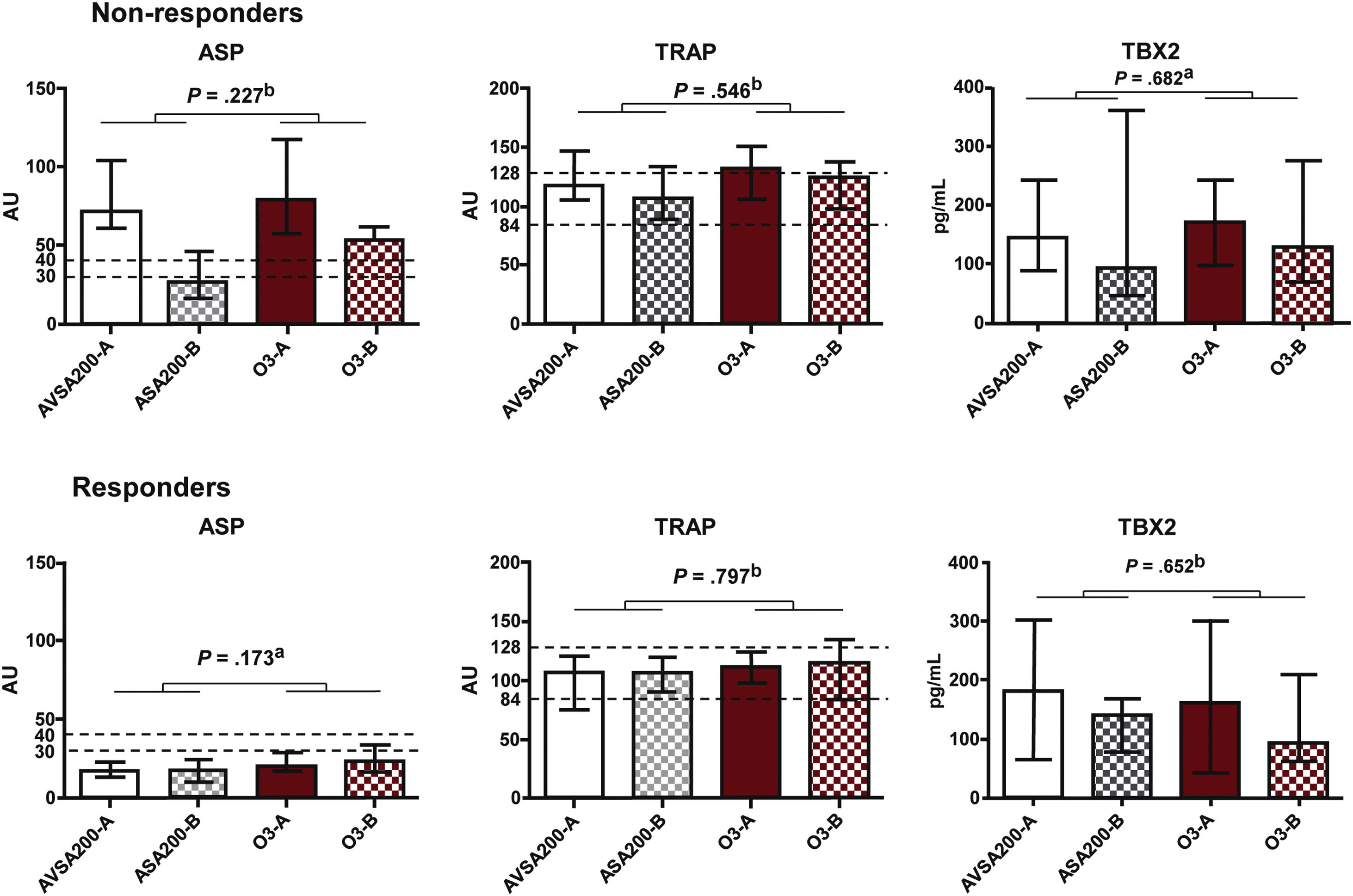
ResultsAnalysis of platelet aggregation and thromboxane dosageFrom the initial analysis of platelet function in the ASA100 group, of all 152 patients, 38 non-responders were detected (25.2%). Subsequent tests of platelet function and plasma TBX2 concentration, including the ASA200 and ASA100O3 treatment groups, showed that the reduction of ASPItest values was more significant (P=.001) in the non-responders’ group (NR) than in the responders’ (R) group (Fig. 3). However, only after treatment do the ASPItest values fall to the levels of responders (<40AU) in the ASA200 group (Fig. 4). In addition, when the R and NR groups were analyzed separately, it was possible to observe a correlation between platelet function test results and TBX2 in the R group of the ASA100O3 treatment (P=.039) (Table 1). By organizing responder and non-responder data by treatment groups (Fig. 5), it can be noted that, although the treatment effect was not statistically different between groups, non-responders who used ASA200 for 30 days had ASPItest values reduced to the level of reclassification as responders (<40AU), which did not occur in the ASA100O3 group.
Mann–Whitney test comparison of the results expressed as a median and interquartile range of the effect of ASA200 and ASA100O3 treatments on platelet function (ASP) and thromboxane B2 (TXB2) production between responder (R) and non-responder (NR) groups at Times A (after 30 days of ASA100 treatment) and B (after 30 days of ASA200 and ASA100O3 treatments).
Effect of treatments, expressed as median and interquartile range values, on platelet function measured by ASPItest (ASP) and TRAPtest (TRAP), and plasma concentration of thromboxane B2 (TBX2) in the groups of responders (R) and non-responders (NR) to acetylsalicylic acid (ASA) at Times A (after 30 days of treatment with ASA 100mg, ASA100) and B (after 30 days of treatment with ASA 200mg, ASA200, or ASA 100mg and omega-3, ASA100O3). a Mann–Whitney test. b Student's t-test.
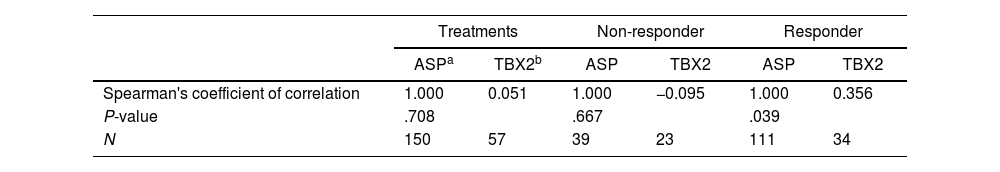
Evaluation of the correlation between treatments and results in ASPItest and plasma thromboxane B2 concentration tests between treatment groups and responders and non-responders.
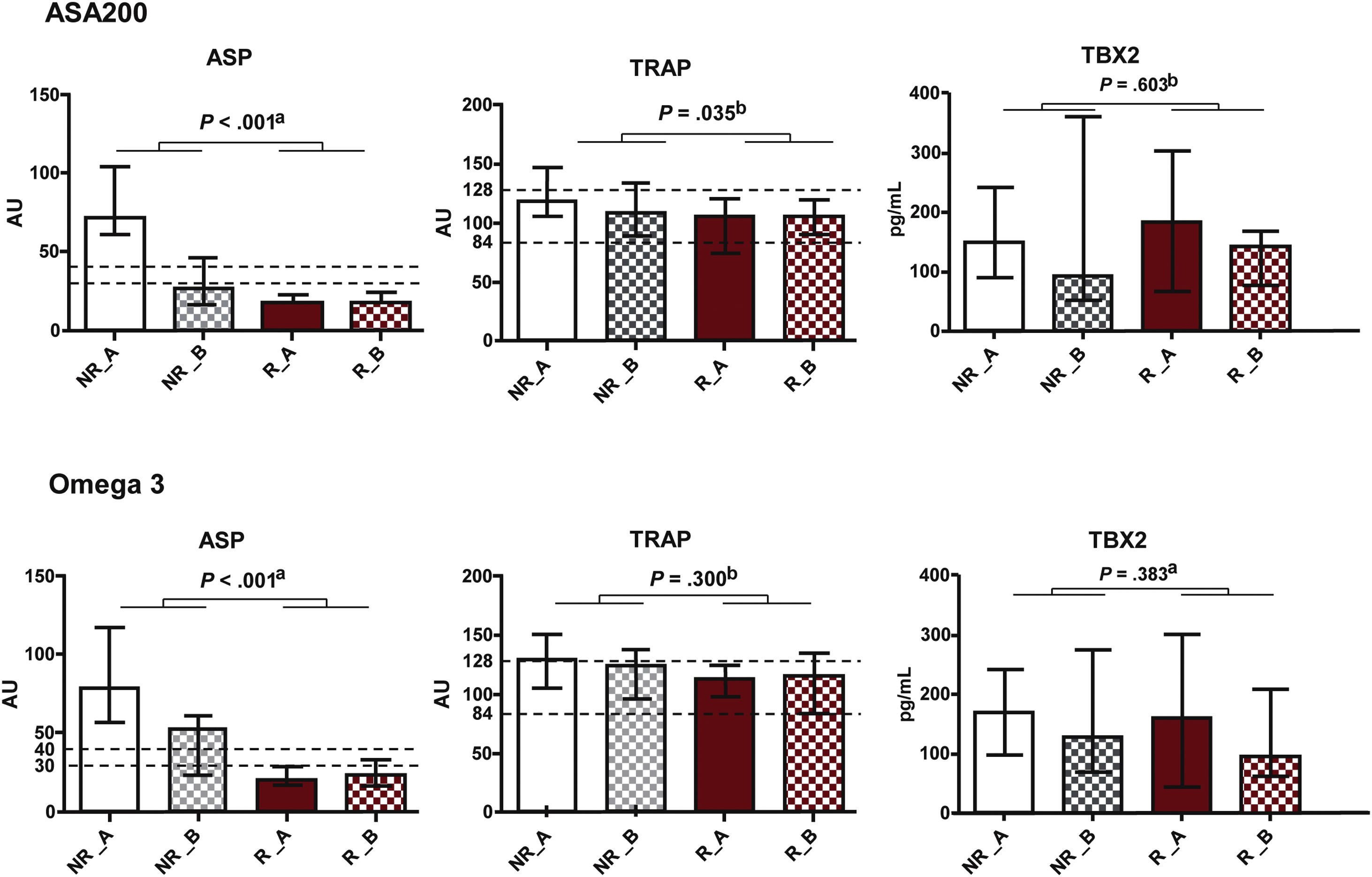
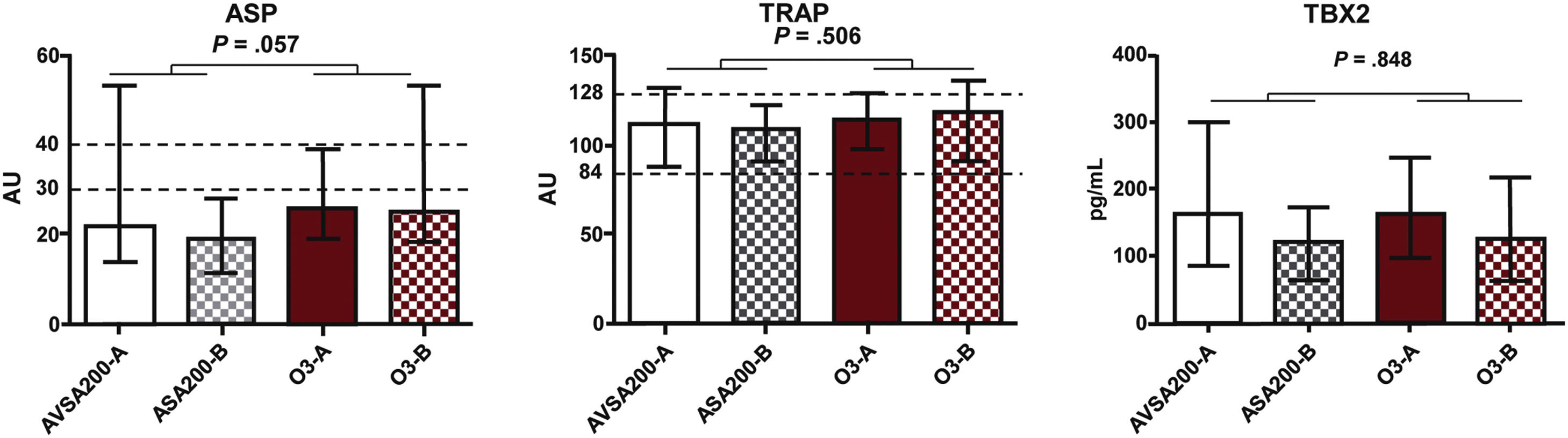
Effect of ASA200 and ASA100O3 treatments, expressed as median and interquartile range values, on platelet function (ASP and TRAP) and plasma concentration of thromboxane B2 (TBX2) at Times A (after 30 days of ASA100 treatment) and B (after 30 days of ASA200 or ASA100O3 treatment). a Mann–Whitney test. b Student's t-test.
There was no statistically significant difference between treatment groups concerning platelet function (ASPItest and TRAPtest) and TBX2 production (Fig. 6). TRAPtest values were within the expected range (84–128), demonstrating that platelet function was preserved in these patients. There was also no correlation between ASPItest results and plasma TBX2 concentration (Table 1).
[CS1]Mann–Whitney test comparison of the results expressed as a median and interquartile range of the effect of ASA200 and ASA100O3 treatments on platelet function (ASP and TRAP) and thromboxane B2 (TBX2) production at Times A (after 30 days of ASA100 treatment) and B (after 30 days of ASA200 or ASA100O3 treatment).
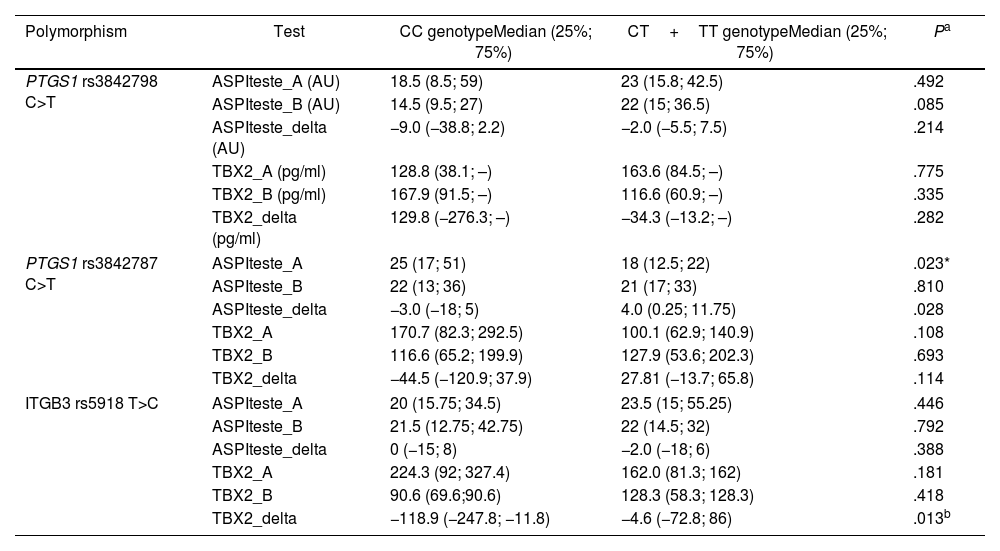
The rare allele of the PTGS1 rs3842787 polymorphism is associated with a worse ASPItest response. In comparison, patients carrying the rare allele of the rs5918 polymorphism in the ITGB3 gene showed a worse response concerning TBX2 concentration after the 30-day treatment (Table 2). No statistical difference was found in the genotypic and allele frequencies of the studied polymorphisms between the R and NR groups and between the ASA200 and ASA100O3 treatments (Table 3 of the supplementary data). Therefore, the rare alleles were equally distributed between R and NR groups and treatment groups. The genotypic distribution of the studied population agreed with the predictions of the Hardy–Weinberg law (Table 4 of the supplementary data).
Effect of rare alleles on ASPItest results and concentration of plasma thromboxane B2.
| Polymorphism | Test | CC genotypeMedian (25%; 75%) | CT+TT genotypeMedian (25%; 75%) | Pa |
|---|---|---|---|---|
| PTGS1 rs3842798 C>T | ASPIteste_A (AU) | 18.5 (8.5; 59) | 23 (15.8; 42.5) | .492 |
| ASPIteste_B (AU) | 14.5 (9.5; 27) | 22 (15; 36.5) | .085 | |
| ASPIteste_delta (AU) | −9.0 (−38.8; 2.2) | −2.0 (−5.5; 7.5) | .214 | |
| TBX2_A (pg/ml) | 128.8 (38.1; –) | 163.6 (84.5; –) | .775 | |
| TBX2_B (pg/ml) | 167.9 (91.5; –) | 116.6 (60.9; –) | .335 | |
| TBX2_delta (pg/ml) | 129.8 (−276.3; –) | −34.3 (−13.2; –) | .282 | |
| PTGS1 rs3842787 C>T | ASPIteste_A | 25 (17; 51) | 18 (12.5; 22) | .023* |
| ASPIteste_B | 22 (13; 36) | 21 (17; 33) | .810 | |
| ASPIteste_delta | −3.0 (−18; 5) | 4.0 (0.25; 11.75) | .028 | |
| TBX2_A | 170.7 (82.3; 292.5) | 100.1 (62.9; 140.9) | .108 | |
| TBX2_B | 116.6 (65.2; 199.9) | 127.9 (53.6; 202.3) | .693 | |
| TBX2_delta | −44.5 (−120.9; 37.9) | 27.81 (−13.7; 65.8) | .114 | |
| ITGB3 rs5918 T>C | ASPIteste_A | 20 (15.75; 34.5) | 23.5 (15; 55.25) | .446 |
| ASPIteste_B | 21.5 (12.75; 42.75) | 22 (14.5; 32) | .792 | |
| ASPIteste_delta | 0 (−15; 8) | −2.0 (−18; 6) | .388 | |
| TBX2_A | 224.3 (92; 327.4) | 162.0 (81.3; 162) | .181 | |
| TBX2_B | 90.6 (69.6;90.6) | 128.3 (58.3; 128.3) | .418 | |
| TBX2_delta | −118.9 (−247.8; −11.8) | −4.6 (−72.8; 86) | .013b | |
A: response to treatment ASA100 (first visit); B: response to treatment ASA200 or ASA100O3 (second visit); delta: B-A. C: cytosine; T: thymine; CC: cytosine homozygous genotype; TT: thymine homozygous genotype; CT: heterozygous genotype.
There was a significant reduction in VLDL and triglyceride values in the ASA200 (P=.005 and P=.002, respectively) and ASA100O3 (P=.006 and P=.004, respectively) groups after 30 days of treatment. However, this reduction was not significantly different between both treatments. It was also possible to observe a significant reduction in the number of monocytes (P=.024) in the ASA100O3 treatment group compared to the ASA200 group.
DiscussionPatients initially classified as non-responders showed a significant reduction in ASPItest values in the analysis of platelet function and plasma TBX2 concentration after 30 days of ASA200 treatment. In this treatment group, ASPItest values dropped to <40AU, reaching the responder classification. This reduction was not detected in the ASA100O3 treatment group. It is important to note that the doses of ASA used in this study were low, agreeing with other studies reporting the use of doses of 50, 100, 325, and 1000mg.21,22
Platelet function was preserved in patients in the ASA200 and ASA100O3 treatment groups, as demonstrated by TRAPtest values, which were within the expected range (84–128). Thus, there was no statistically significant difference in platelet function measured by the ASPItest and TRAPtest between the ASA200 and ASA100O3 treatment groups regarding the TBX2 production. Interestingly, patients in both groups had reduced VLDL and triglyceride values after 30 days of treatment, with no significant difference between ASA200 and ASA100O3 treatments. Also, in the ASA100O3 group, there was a decrease in monocytes, which may be related to the anti-inflammatory action of omega-3 on atherosclerotic plaque. These results are encouraging in light of evidence from studies reporting a 30% reduction in inflammatory activity after four weeks of treatment with omega-3 fatty acid intake.23,24
The correlation between the results of the platelet function test and TBX2 production of the responder's group agrees with Kidson-Gerber et al.,25 which show that measuring TBX2 levels is a direct and easy-to-apply method to analyze the effect of ASA on platelets. Hence, this method has an adequate specificity in measuring the ability of platelets to produce thromboxane A2 and, consequently, directly measures the effect of ASA on platelet function.25 These platelet response analysis techniques are accessible to medical practice. In addition, our results show that monitoring the patient's response to anticoagulant treatment is fundamental in clinical practice. In this way, the physician can timely identify a non-responder patient, considering the techniques used in our study.
Analysis of lack of response to ASA should not be limited to its pharmacological effect on COX-1 thromboxane-dependent inhibition. Treatment failure should also be analyzed concerning other events occurring under the use of ASA or when there is inhibition of platelet activation. Environmental factors, gender, obesity, smoking, drug interactions (NSAIDs), and conditions associated with increased platelet activity, such as exercise, dyslipidemia, systemic hypertension, and acute diseases, can modify the inhibitory action of ASA on platelets.26 Therefore, specific tests for the effect of ASA, such as those of TBX2 levels, are adequate for differentiating between an insufficient pharmacological action of ASA or other causes of treatment failures in non-responders. In patients who respond to ASA in TBX2 tests and on aggregometer platforms, ASA's inability to inhibit A2-dependent thromboxane production and high innate platelet reactivity may occur together, thus confirming, in these cases, a reduced effect of ASA in preventing cardiovascular events. Therefore, in clinical practice, it is helpful to use a test of the pharmacological effect of ASA (serum TBX2 level) and a platelet function test (Multiplate).
Regarding the polymorphisms of the PTGS 1 – (COX-1) gene, the rare allele rs3842787 was found to be associated with a worse response in ASPItest in non-responders. On the other hand, patients with the rare allele rs5918 of the ITGB 3 gene (GPIIIa) have a worse response regarding the concentration of TBX2 after 30 days of treatment. Various candidate genes have been suggested to explain the genetic polymorphisms impacting the analysis of interindividual response variation to ASA.27–29 A meta-analysis of 31 studies, including 50 polymorphisms of 11 genes and 2834 patients, reported the polymorphism in the rs5918 region of the ITGB3 gene of the GPIIIa platelet receptor as the most frequently investigated and reported in association with ASA resistance.30 These authors showed no association of polymorphism in the rs3842787 region of the COX-1 gene (A 842G/C5OT) with ASA resistance. Maree et al.31 genotyped 144 patients with coronary artery disease and, of the five polymorphisms studied, showed a significant effect of antiplatelet modulation of ASA for the C50T variant (rs3842787). Similar observations were made by Lepäntalo et al.32 Su et al.33 showed that the 807T (GPIIIa) – r55918 allele confers 3.8 times more resistance to the effect of ASA in 200 patients with atherosclerosis than in 807 homozygotes from a Chinese population.
ASA resistance analyzed by genetic variants considers that polymorphisms vary according to ethnicity. Thus, Wagner et al.34 detected higher resistance to ASA for Europeans with rs3842787 and Africans with rs3842792. In the Chinese population, gene polymorphism rs5918 strongly correlates with ASA resistance.34 More specifically, Shiffman et al.35 showed that carriers of an SNP related to plasma lipoprotein levels benefit more from treatment with ASA than non-carriers. Studies like these may lead to a personalized genetic profile that more accurately identifies which patients will benefit most from using ASA.36 Despite their usefulness, the cost of genetic analyses may limit their application in particular clinical practices. However, these analyses are increasingly widespread and accessible to public and private health centers. Therefore, genetic analyses can become valuable tools to analyze SNP polymorphisms in non-responders and thus guide the most appropriate treatment for each case.
The efficacy and safety of ASA have been extensively analyzed in apparently normal populations at low, moderate, or high risk for cardiocirculatory events and populations with diseases with or without a history of AMI and/or stroke.37–39 Specifically, a meta-analysis on primary atherosclerosis prevention studies showed that ASA reduced the total number of cardiovascular events by 10%, primarily by reducing nonfatal AMI (20%).40 However, the reduction in mortality from cardiovascular causes and neoplasms was negligible, 1% and 7%, respectively. Bleeding occurred in 30% of individuals using ASA. These “modest benefits” and the high risk of bleeding do not justify the routine use of ASA in the primary prevention of cardiovascular diseases. Hence, there is a need to identify population groups that will benefit from treatment and establish adequate doses, aiming at therapeutic effectiveness and low risk of bleeding. In our study, patients with the s384787 allele had no higher incidence of thrombosis or bleeding. Still, none of these clinical signs were detected or reported by patients from both treatment groups during clinical evaluation at Time B. However, new studies will be developed to analyze these clinical signs in more detail.
LimitationsSome limitations of our study are related to a relatively small sample size, the usage of 1g of omega-3, despite literature describing 1–4g per day, and the lack of in vitro analysis of platelet aggregation on diabetic patients that were not the subject of this study. In addition, adherence to medication intake was monitored during clinical evaluations and by telephone. Therefore, our results should be carefully interpreted since they originated from a single-center study on a limited number of patients. This limited n may influence the results of statistical analyses. However, our results show helpful information for clinical practice and the design of more complex, multi-center studies on more patients. Among the studies derived from our research, we can include the analysis of temporal variation in platelet activity, stratified studies in responders and non-responders, or analyses of isolated non-responders. These future studies may apply our methodology to assess, for example, the response of patients with atrial fibrillation to oral anticoagulant administration.
ConclusionsCombining platelet function analysis and genetic testing is key to identifying and classifying ASA non-responder patients. In this way, the best available treatment can be administered to each patient. Within these possibilities, we recommend considering ASA200 treatment for 30 days to reduce the ASPItest values of non-responder patients down to the responder level. Interestingly, the use of the two treatments in the present study showed no statistically significant difference between the two treated groups regarding absence of hematuria and bleeding, constituting safe treatment alternatives. In addition, treatment with 1g/day of omega-3 fatty acid, one of the lowest doses concerning those reported in the literature,41 reduced the number of monocytes. The detection of the rs384787 alleles associated with the worst response to ASPItest and the rs5918 allele to the worst response in the TBX2 test, combined with the therapeutic findings described, reinforces the usefulness of pharmacogenetics, enabling personalized patient treatment, and avoiding the undesirable effects of gastrointestinal symptoms and bleeding of the ASA.
Antiplatelet agents like ASA have a central function in the prevention of atherothrombosis. However, some patients using ASA continue to suffer atherothrombotic events, and approximately 20% will have some recurrent vascular event. Some of these side effects, such as bleeding, raising the debate about creating a specific therapy for each patient. In addition, the combined use of other drugs, such as omega-3, can potentiate the antiaggregant effects of ASA without its side effects.
Does it contribute anything new?We show that patients initially classified as non-responders obtain a significant reduction in ASPItest values and plasma TBX2 concentration after 30 days of ASA200 treatment. In addition, there is a significant reduction in VLDL and triglyceride values in the ASA200 and ASA100O3 treatments. The absence of hematuria and bleeding confirms both treatments as safe therapeutic alternatives. We detected rare alleles associated with worst responses to ASPItest and TBX2 concentration analysis.
This work received support from the Adib Jatene Foundation, Av. Dr. Dante Pazzanese, 500 – Vila Mariana, São Paulo, Brasil – CEP 04014-002.
Authors’ contributionsEach author contributed significantly to the submitted work regarding the research conception (M.M. Cartocci, A. Guerra de Moraes Rego Sousa, and D. Armaganijan), data analyses (M.M. Cartocci and M.H. Hirata), and writing of the manuscript (M.M. Cartocci, A. Guerra de Moraes Rego Sousa, and D. Armaganijan, M. Batlouni, and M.H. Hirata).
Conflicts of interestNone.
We thank the Adib Jatene Foundation for the financial support to conduct genetic and platelet aggregation analyses and the Associac¸ao Fundo de Incentivo a Pesquisa for collaborating on the biochemical tests. We also thank two anonymous reviewers for their valuable comments on our manuscript.










![[CS1]Mann–Whitney test comparison of the results expressed as a median and interquartile range of the effect of ASA200 and ASA100O3 treatments on platelet function (ASP and TRAP) and thromboxane B2 (TBX2) production at Times A (after 30 days of ASA100 treatment) and B (after 30 days of ASA200 or ASA100O3 treatment). [CS1]Mann–Whitney test comparison of the results expressed as a median and interquartile range of the effect of ASA200 and ASA100O3 treatments on platelet function (ASP and TRAP) and thromboxane B2 (TBX2) production at Times A (after 30 days of ASA100 treatment) and B (after 30 days of ASA200 or ASA100O3 treatment).](https://static.elsevier.es/multimedia/26051532/0000005800000004/v2_202310271217/S2605153223002510/v2_202310271217/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w97tFqh1kTBJPZC4W/at16EQ=)



