To describe the prevalence, clinical profile, and prognostic significance of interatrial block (IAB) in heart failure (HF) inpatients.
MethodsWe included prospectively 557 hospitalized HF patients with sinus rhythm from nationwide registry. Partial IAB was defined as a P wave duration ≥120ms, and advanced IAB as a P wave duration ≥120ms with biphasic morphology in inferior electrocardiogram (ECG) leads. Clinical, blood test, and echocardiographic data were analyzed in a Cox regression to determine the impact of IAB at discharge on prognosis. The primary outcome was a composite of mortality or readmission for HF at 1-, 6-, and 12-month.
ResultsNormal P wave, partial, and advanced IAB were identified in the discharge ECG in 336 (60.3%), 118 (21.1%), and 103 (18.5%) patients, respectively. The independent factors related to IAB at discharge were previous history of HF (OR, 1.78; 95%CI, 1.23–2.57), valvular HF etiology (OR, 1.88; 95%CI, 1.18–3.00), heart rate at admission (OR, 1.10 per 10 beats; 95%CI, 1.03–1.17) and left atrial diameter (OR, 1.24 per 5mm; 95%CI, 1.11–1.38). The incidence of 1-, 6-, and 12-month mortality/readmission for HF was 4.8%, 7.7%, and 33.0% in normal P wave, 1.7%, 7.6%, and 33.2% in partial IAB, and 3.9%, 9.7%, and 36.9% in advanced IAB. Multivariable analysis showed no significant effect of IAB in major acute cardiovascular events.
ConclusionsIAB is found in 40% of patients in sinus rhythm discharged after a HF hospitalization. This ECG pattern at discharge does not imply a greater risk of readmission or death during the first year.
Describir la prevalencia, el perfil clínico y el significado pronóstico del bloqueo interauricular (BIA) en pacientes hospitalizados por insuficiencia cardiaca (IC).
MétodosEstudio de cohortes que incluye a 557 pacientes hospitalizados por IC de un registro nacional. El BIA parcial se definió como una onda p≥120ms, y BIA avanzado si, además presentaba una morfología bifásica en las derivaciones inferiores del electrocardiograma. Se analizaron variables ecocardiográficas, analíticas y clínicas para determinar el impacto pronóstico del BIA al alta en un modelo de Cox. El evento principal fue un combinado de muerte o reingreso por IC a 1, 6 y 12 meses.
ResultadosAl alta, la presencia de una onda P normal, BIA parcial y avanzado se detectó en 336 (60,3%), 118 (21,1%) y 103 (18,5%) pacientes, respectivamente. Los factores independientes de la presencia de BIA fueron IC previa (OR: 1,78; IC95%: 1,23-2,57), etiología valvular (OR: 1,88; IC95%: 1,18-3,00), frecuencia cardiaca al ingreso (OR: 1,10 por 10 latidos; IC95%: 1,03-1,17) y el diámetro auricular izquierdo (OR: 1,24 por 5mm; IC95%: 1,11-1,38). La incidencia del evento combinado a 1, 6 y 12 meses fue del 4,8, 7,7 y 33,0% para los sujetos con P normal; 1,7, 7,6, y 33,2% en BIA parcial, y 3,9, 9,7, y 36,9% en aquellos con BIA avanzado. La presencia del BIA al alta no se identificó como predictor para el evento principal.
ConclusionesHasta el 40% de los pacientes en ritmo sinusal presentan algún grado de BIA al alta de un episodio de IC. Este patrón no supuso un mayor riesgo de reingreso por IC o muerte durante el primer año de seguimiento.
Interatrial block (IAB) is defined as impaired conduction between the right and the left atrium and is reflected by prolonged P wave duration exceeding 120ms on surface electrocardiogram (ECG).1 IAB is correlated with atrial enlargement and dysfunction,2 and is strongly associated with atrial tachyarrhythmia, particularly atrial fibrillation,3,4 as well as embolic stroke.5 Although IAB can be easily identified in routinely performed ECGs, it remains a poorly recognized cardiac conduction disorder6 in daily clinical practice.
A careful ECG analysis is useful in order to obtain essential information for the management and prognostic evaluation of patients with heart failure (HF). Impairment of interatrial conduction may occur in patients with HF due to structural anomaly and/or increased atrial filling pressure. Moreover, atrial conduction delay has been described in patients with chronic HF and it has even been proposed as a potential therapeutic target in selected patients.7 However, data are lacking regarding the frequency and characteristics of IAB in patients hospitalized for an episode of acute HF.
Thus, the objective of our study was to describe the prevalence, clinical profile, and short- and mid-term prognosis of IAB in patients admitted for HF.
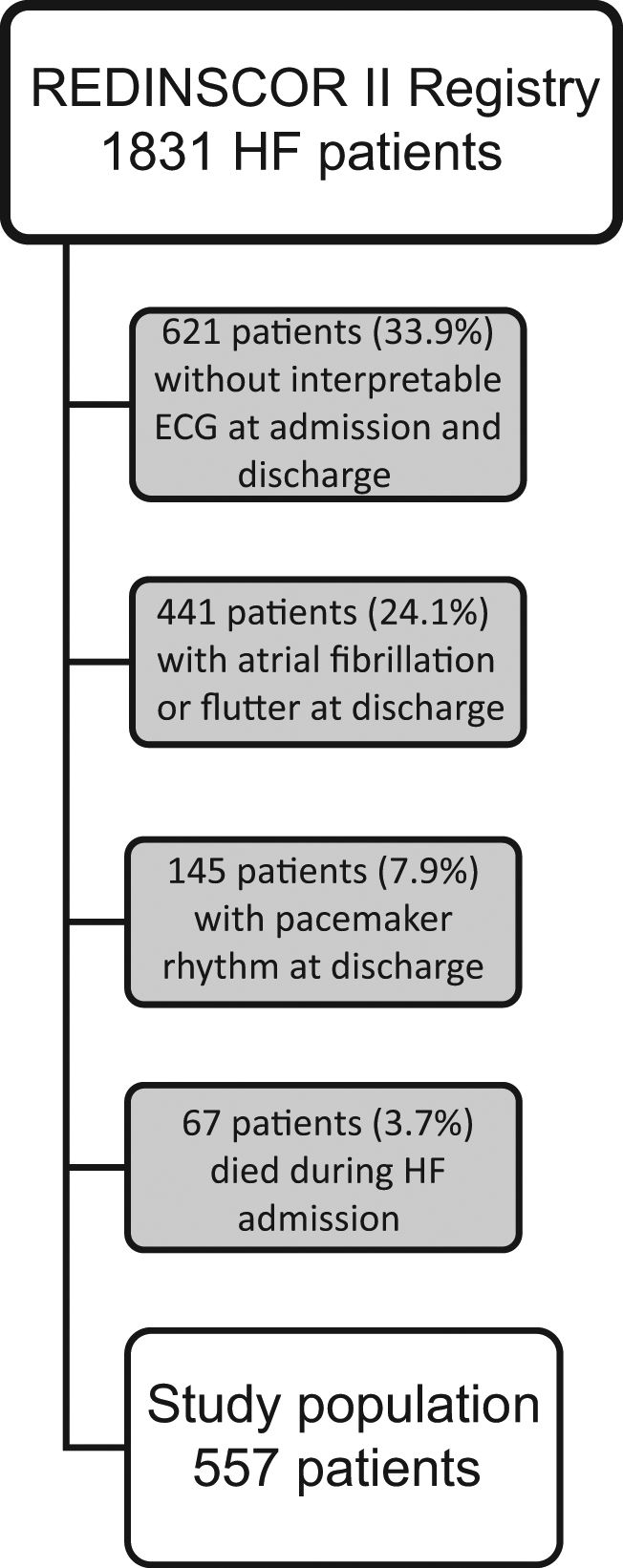
MethodsStudy populationThe REDINSCOR II study is a national, multicenter, prospective registry, which enrolled consecutively 1831 patients from 20 Spanish secondary and tertiary hospitals since October 2013 to December 2014. Inclusion criteria were: (a) age older than 18 years; (b) acute HF as the main cause for admission; and (c) hospitalization≥24h in the Cardiology Department. Exclusion criteria were: (a) HF episode secondary to ST-segment elevation acute coronary syndrome; (b) end-stage disease with a life expectancy<1 year; and (c) any condition that would prevent an appropriate follow-up. HF was diagnosed in accordance with current HF guidelines.8 For this analysis, only were included those patients discharged alive with available 12-lead ECG recordings both at admission and discharge (n=1143). Out of 1143, a total of 441 patients were excluded for atrial fibrillation or flutter at discharge, and 145 patients for atrial paced rhythm. Therefore, the final sample size of our study was 557 patients (Fig. 1). The study complies with the Declaration of Helsinki and the protocol was approved by the Ethics Committees of each participating center. All patients gave written informed consent.
Study variablesData were collected using specifically designed web forms and quality controls were undertaken periodically. The following clinical variables were gathered at study inclusion and at hospital discharge: demographic and previous clinical data, case history and physical examination, chest x-rays, ECG, echocardiogram, laboratory blood tests, and pharmacological and non-pharmacological treatments. A detailed list of the study variables is provided in the supplementary data. Standard criteria were used to define the clinical variables. Left ventricular ejection fraction was categorized according to the recent HF European guidelines: HF with reduced ejection fraction (HFrEF<40%), HF with mid-range ejection fraction (HFmrEF 40–49%), and HF with preserved ejection fraction (HFpEF≥50%). The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) method.
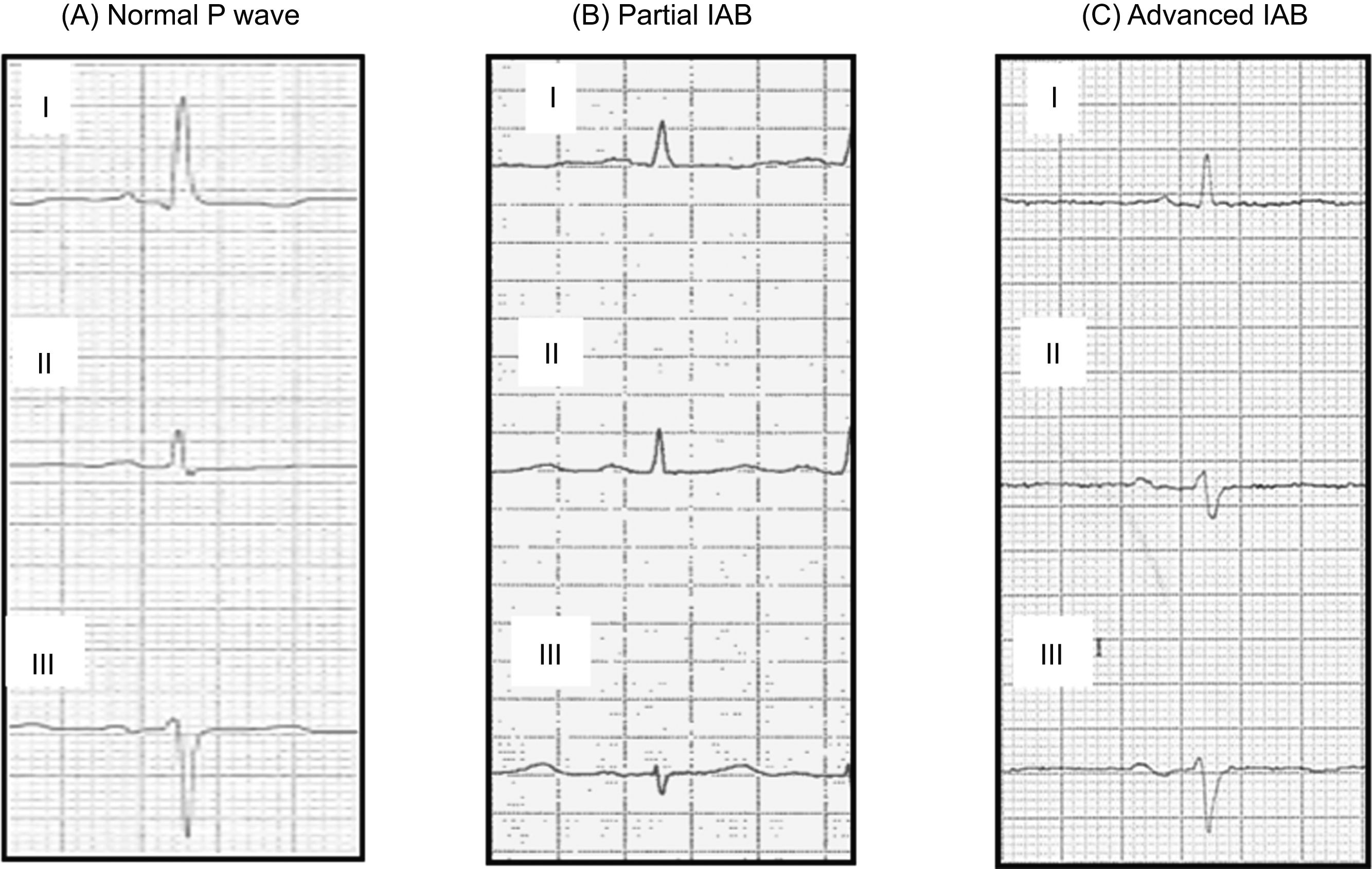
ECG analysisA standard 12-lead ECG (filter 150Hz, 25mm/s, 10mm/mV) was obtained in all patients at admission and prior to discharge. All ECG recordings were scanned at a minimum resolution of 600dpi. Images were analyzed using the GeoGebra 5.0 software. Digital calipers were used to measure P wave duration, determined from the earliest to the latest P wave deflection in any frontal lead (Fig. 1 of the supplementary data). The ECG analysis was performed by four blinded independent observers, and when there was doubt, a fifth expert observer was consulted. P wave morphology was assessed in leads II, III, and aVF. Bimodal (positive/negative) P wave morphology was defined as an initial portion that deviates upwards from the baseline and a final portion that deviates downwards from the baseline. Normal atrial conduction was considered as a P wave<120ms regardless of morphology, partial IAB was defined as a P wave≥120ms without bimodal morphology, and advanced IAB was defined as a P wave≥120ms with bimodal morphology, as described in a consensus document (Fig. 2).1
Follow-up and outcomesIn addition to the specific clinical follow-up needed by the patient, the vital status was checked at 1, 6 and 12 months after discharge. We alternatively used telephone interviews, the clinical records of the hospital and primary care, or the institutional death registries. Thirty patients (5%) were lost during the follow-up and were censored for the statistical analysis. The primary outcome was a combined clinical event including overall death (including heart transplant) or hospitalization for worsening of HF (major acute cardiovascular events [MACE]) at 1-, 6-, and 12-month after discharge. Secondary outcomes were each one of clinical events separately. The reported events were reviewed by an ad hoc committee.
Statistical analysisContinuous variables are expressed as mean±standard deviation (SD) or as median [interquartile range] whenever appropriate. Differences in continuous variables were tested by the analysis of variance (ANOVA), Student's t test, or Wilcoxon signed rank test for independent samples. Categorical variables were presented as frequency and percentage. Differences in the categorical variables were assessed by the Chi-square test or by Fisher's exact test.
We conducted a binary logistic regression analysis to identify the factors related with the presence of IAB at discharge. A backward stepwise method was used with a P<.05 for the inclusion or deletion criteria. We performed survival analyses using Cox regression models weighted by IPTW (inverse probability of treatment weighting)9 method to assess the relationship of type of IAB with the primary and secondary outcomes. IPTW was used to balance groups and minimize the bias arising from an observational study of the effect of IAB type. IPTW balances the baseline group characteristics for a set of defined variables, allowing analysis of the effect of an intervention or factor (Fig. 2 of the supplementary data). This method was done using the IPW statistical package in R. The following independent variables were evaluated: age, previous HF diagnosis, HF evolution in years, number of previous HF admissions, valvular HF etiology, left atrial diameter, N-terminal pro-B-type natriuretic peptide and growth differentiation factor 15 (GDF-15) values at discharge, and heart rate and left bundle branch block at discharge. These variables were selected by using a statistical criterion (P<.1) or clinical relevance. The discrimination of each model was assessed using the C-statistic index (0.627 for “Partial IAB” and 0.727 for “Advanced IAB”, respectively). Kaplan–Meier survival plots were generated for the cohort, with comparison by the log-rank test. The proportional hazard assumption was evaluated by the Shoenfeld residuals test. A two-sided P<.05 was considered statistically significant. Missing data were imputed using the ‘mice’ package in R (Multivariate Imputation by Chained Equations) whenever necessary (m=1) applying the “predictive mean matching” criteria. All analysis were performed using R (v. 3.5.2) and STATA software (v. 13.1).
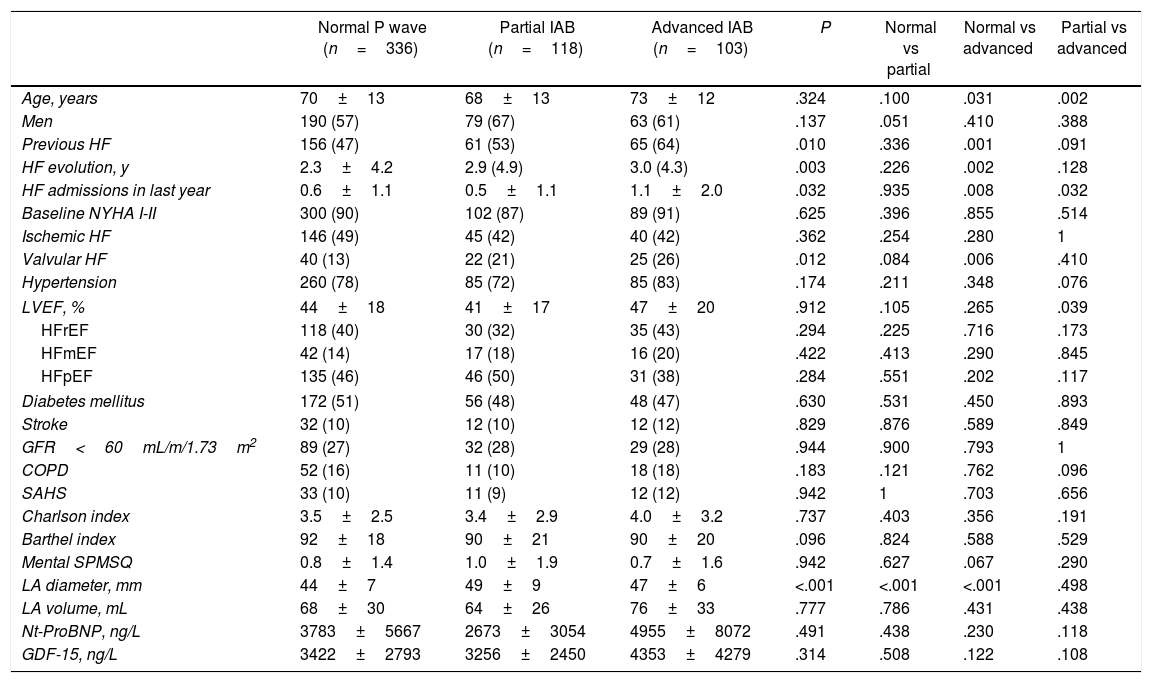
ResultsPrevalence of IAB and clinical profile of patients according to ECG pattern at dischargeOut of 557 patients discharged in sinus rhythm, 336 (60.3%) had normal atrial conduction, 118 partial IAB (21.2%), and 103 (18.5%) advanced IAB. The clinical characteristics of these patients according to ECG pattern at discharge are shown in Table 1. Briefly, patients presenting advanced IAB were older, with more frequent previous history of HF of longer evolution and marked by recurrent admissions than those with normal P wave. Valvular etiology was significantly more frequent in patients with IAB, especially in those with advanced IAB. Depressed left ventricular ejection fraction was more frequent in normal P wave. Parameters related to pressure and/or volume overload such as left atrial size, N-terminal pro-B-type natriuretic peptide, and GDF-15 levels at discharge were higher in patients with advanced IAB than in the other groups. Table 1 of the supplementary data compares the clinical characteristics between the study population and excluded patients.
Clinical characteristics according to electrocardiogram pattern at discharge.
| Normal P wave (n=336) | Partial IAB (n=118) | Advanced IAB (n=103) | P | Normal vs partial | Normal vs advanced | Partial vs advanced | |
|---|---|---|---|---|---|---|---|
| Age, years | 70±13 | 68±13 | 73±12 | .324 | .100 | .031 | .002 |
| Men | 190 (57) | 79 (67) | 63 (61) | .137 | .051 | .410 | .388 |
| Previous HF | 156 (47) | 61 (53) | 65 (64) | .010 | .336 | .001 | .091 |
| HF evolution, y | 2.3±4.2 | 2.9 (4.9) | 3.0 (4.3) | .003 | .226 | .002 | .128 |
| HF admissions in last year | 0.6±1.1 | 0.5±1.1 | 1.1±2.0 | .032 | .935 | .008 | .032 |
| Baseline NYHA I-II | 300 (90) | 102 (87) | 89 (91) | .625 | .396 | .855 | .514 |
| Ischemic HF | 146 (49) | 45 (42) | 40 (42) | .362 | .254 | .280 | 1 |
| Valvular HF | 40 (13) | 22 (21) | 25 (26) | .012 | .084 | .006 | .410 |
| Hypertension | 260 (78) | 85 (72) | 85 (83) | .174 | .211 | .348 | .076 |
| LVEF, % | 44±18 | 41±17 | 47±20 | .912 | .105 | .265 | .039 |
| HFrEF | 118 (40) | 30 (32) | 35 (43) | .294 | .225 | .716 | .173 |
| HFmEF | 42 (14) | 17 (18) | 16 (20) | .422 | .413 | .290 | .845 |
| HFpEF | 135 (46) | 46 (50) | 31 (38) | .284 | .551 | .202 | .117 |
| Diabetes mellitus | 172 (51) | 56 (48) | 48 (47) | .630 | .531 | .450 | .893 |
| Stroke | 32 (10) | 12 (10) | 12 (12) | .829 | .876 | .589 | .849 |
| GFR<60mL/m/1.73m2 | 89 (27) | 32 (28) | 29 (28) | .944 | .900 | .793 | 1 |
| COPD | 52 (16) | 11 (10) | 18 (18) | .183 | .121 | .762 | .096 |
| SAHS | 33 (10) | 11 (9) | 12 (12) | .942 | 1 | .703 | .656 |
| Charlson index | 3.5±2.5 | 3.4±2.9 | 4.0±3.2 | .737 | .403 | .356 | .191 |
| Barthel index | 92±18 | 90±21 | 90±20 | .096 | .824 | .588 | .529 |
| Mental SPMSQ | 0.8±1.4 | 1.0±1.9 | 0.7±1.6 | .942 | .627 | .067 | .290 |
| LA diameter, mm | 44±7 | 49±9 | 47±6 | <.001 | <.001 | <.001 | .498 |
| LA volume, mL | 68±30 | 64±26 | 76±33 | .777 | .786 | .431 | .438 |
| Nt-ProBNP, ng/L | 3783±5667 | 2673±3054 | 4955±8072 | .491 | .438 | .230 | .118 |
| GDF-15, ng/L | 3422±2793 | 3256±2450 | 4353±4279 | .314 | .508 | .122 | .108 |
COPD, chronic obstructive pulmonary disease; GDF, growth differentiation factor; GFR, glomerular filtration rate; HF, heart failure; HFmEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IAB, interatrial block; LA, left atrium; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; Nt-ProBNP, N-terminal pro-B-type natriuretic peptide; SAHS, sleep apnea hypoventilation syndrome; SPMSQ, short portable mental status questionnaire; SD, standard deviation.
Data are expressed as no. (%) or mean±standard deviation.
After a binary logistic regression analysis, the independent factors related with the presence of IAB at discharge were previous history of HF (OR, 1.68; 95%CI, 1.17–2.41), valvular HF etiology (OR, 1.81; 95%CI, 1.14–2.87), heart rate at admission (OR, 1.10 per 10 beats; 95%CI, 1.02–1.17) and left atrial diameter (OR, 1.20 per 5mm; 95%CI, 1.08–1.34). Fig. 3 of the supplementary data shows the calibration plot of the model.
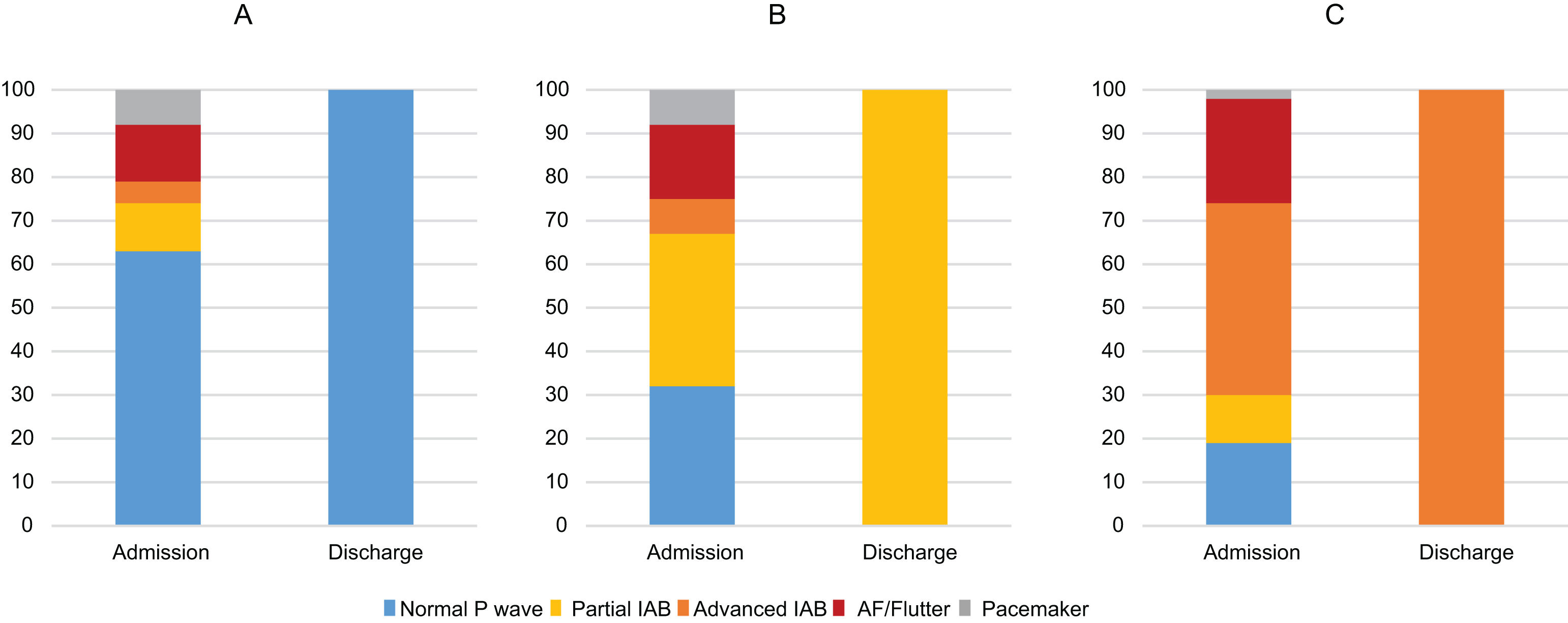
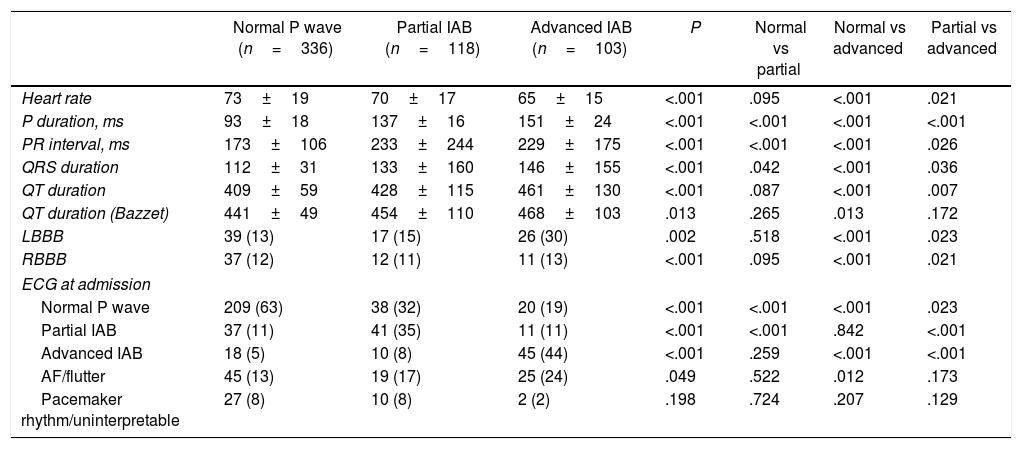
ECG characteristics and evolving changes during compensation of acute HFTable 2 summarizes the ECG characteristics of the study population according to the ECG pattern at discharge. The mean duration of the QRS complex and the presence of both right or left bundle branch blocks was higher in patients with advanced IAB than in other groups. Out of 429 patients in sinus rhythm at admission, the prevalence of partial and advanced IAB at admission was 21% (89 patients) and 17% (73), respectively. Interestingly, Fig. 3 shows the ECG pattern of patients on admission according to ECG pattern at discharge. While the majority of patients with normal atrial conduction at discharge already presented a normal P wave pattern at admission (Fig. 3A), the proportion of AF at admission was progressively increased until the highest value in the group with advanced IAB pattern at discharge (Fig. 3B, C).
Electrocardiogram characteristics according to electrocardiogram pattern at discharge.
| Normal P wave (n=336) | Partial IAB (n=118) | Advanced IAB (n=103) | P | Normal vs partial | Normal vs advanced | Partial vs advanced | |
|---|---|---|---|---|---|---|---|
| Heart rate | 73±19 | 70±17 | 65±15 | <.001 | .095 | <.001 | .021 |
| P duration, ms | 93±18 | 137±16 | 151±24 | <.001 | <.001 | <.001 | <.001 |
| PR interval, ms | 173±106 | 233±244 | 229±175 | <.001 | <.001 | <.001 | .026 |
| QRS duration | 112±31 | 133±160 | 146±155 | <.001 | .042 | <.001 | .036 |
| QT duration | 409±59 | 428±115 | 461±130 | <.001 | .087 | <.001 | .007 |
| QT duration (Bazzet) | 441±49 | 454±110 | 468±103 | .013 | .265 | .013 | .172 |
| LBBB | 39 (13) | 17 (15) | 26 (30) | .002 | .518 | <.001 | .023 |
| RBBB | 37 (12) | 12 (11) | 11 (13) | <.001 | .095 | <.001 | .021 |
| ECG at admission | |||||||
| Normal P wave | 209 (63) | 38 (32) | 20 (19) | <.001 | <.001 | <.001 | .023 |
| Partial IAB | 37 (11) | 41 (35) | 11 (11) | <.001 | <.001 | .842 | <.001 |
| Advanced IAB | 18 (5) | 10 (8) | 45 (44) | <.001 | .259 | <.001 | <.001 |
| AF/flutter | 45 (13) | 19 (17) | 25 (24) | .049 | .522 | .012 | .173 |
| Pacemaker rhythm/uninterpretable | 27 (8) | 10 (8) | 2 (2) | .198 | .724 | .207 | .129 |
AF, atrial fibrillation; IAB, interatrial block; LBBB, left bundle branch block; RBBB, right bindle branch block.
Data are expressed as no. (%) or mean±standard deviation.
Electrocardiogram pattern of patients on admission according to electrocardiogram pattern at discharge. While the majority of patients with normal atrial conduction at discharge already presented a normal P wave pattern at admission (A), the proportion of AF at admission was progressively increased until the highest value in the group with advanced interatrial block (IAB) pattern at discharge (B,C). AF: atrial fibrillation.
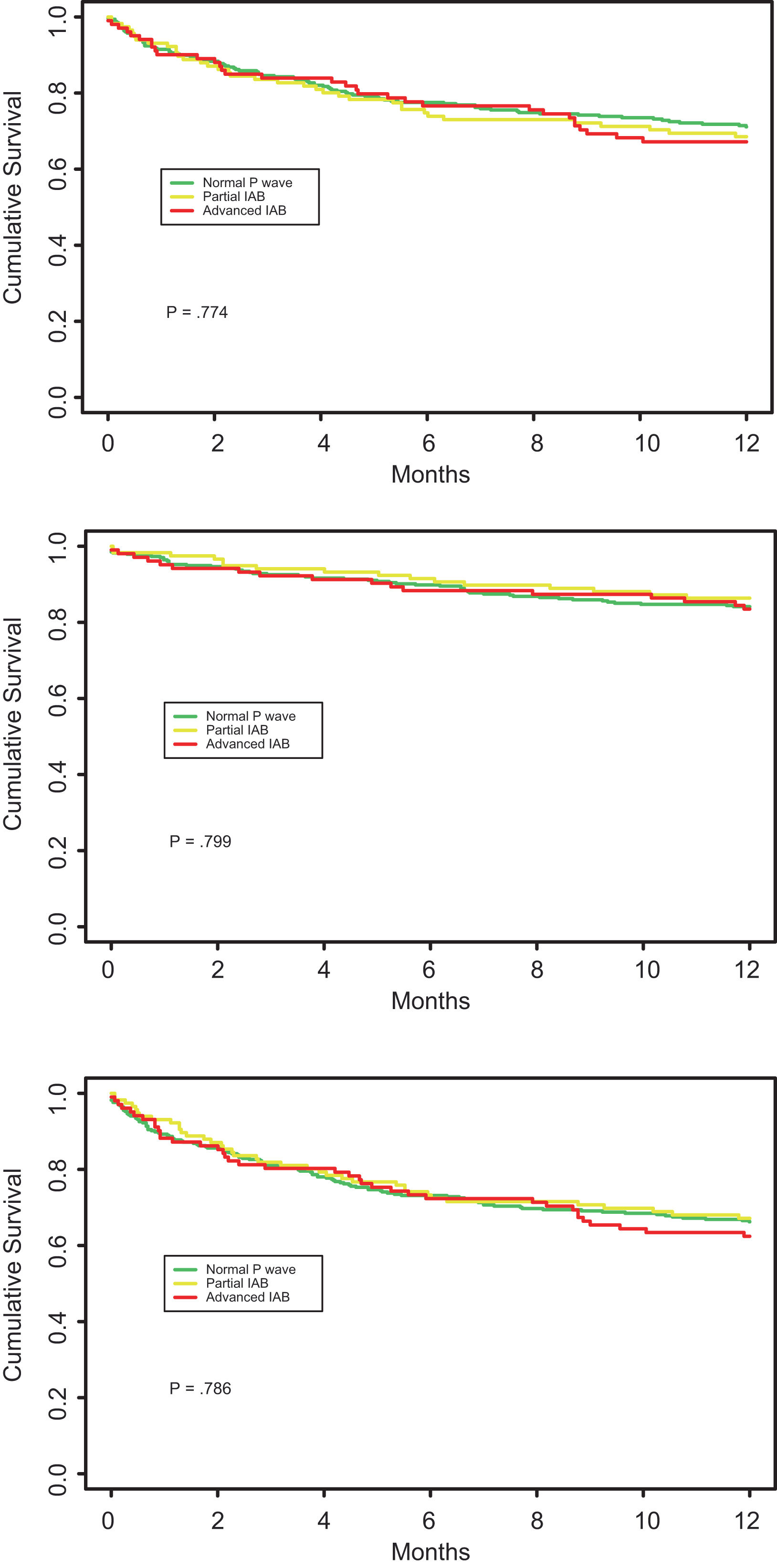
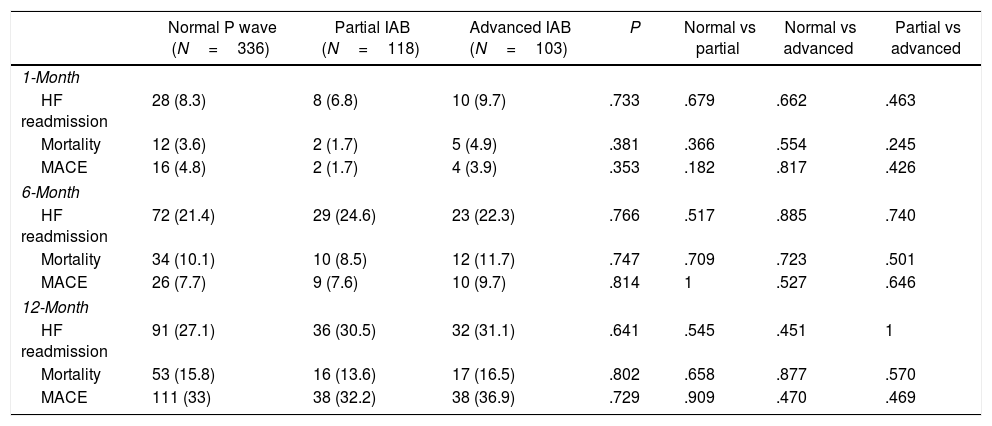
Overall, the HF readmission rate at 1-, 6-, and 12-month was 3.4%, 10.1%, and 15.4%, respectively. The mortality rate at 1-, 6-, and 12-month was 7.9%, 22.2%, and 28.5%. There were no significant differences among the rates of primary and secondary outcomes at 1 month and 6 months in the 3 groups, with only a slight tendency for patients with advanced IAB to present a worse prognosis at 12 months than patients with normal P wave. Table 3 resumes the clinical outcomes of the study population according to ECG pattern at discharge. Fig. 4 shows the Kaplan–Meier survival curves for 12-month HF readmission, 12-month mortality, and 12-month MACE according to the ECG pattern at discharge.
Clinical outcomes according to electrocardiogram pattern at discharge.
| Normal P wave (N=336) | Partial IAB (N=118) | Advanced IAB (N=103) | P | Normal vs partial | Normal vs advanced | Partial vs advanced | |
|---|---|---|---|---|---|---|---|
| 1-Month | |||||||
| HF readmission | 28 (8.3) | 8 (6.8) | 10 (9.7) | .733 | .679 | .662 | .463 |
| Mortality | 12 (3.6) | 2 (1.7) | 5 (4.9) | .381 | .366 | .554 | .245 |
| MACE | 16 (4.8) | 2 (1.7) | 4 (3.9) | .353 | .182 | .817 | .426 |
| 6-Month | |||||||
| HF readmission | 72 (21.4) | 29 (24.6) | 23 (22.3) | .766 | .517 | .885 | .740 |
| Mortality | 34 (10.1) | 10 (8.5) | 12 (11.7) | .747 | .709 | .723 | .501 |
| MACE | 26 (7.7) | 9 (7.6) | 10 (9.7) | .814 | 1 | .527 | .646 |
| 12-Month | |||||||
| HF readmission | 91 (27.1) | 36 (30.5) | 32 (31.1) | .641 | .545 | .451 | 1 |
| Mortality | 53 (15.8) | 16 (13.6) | 17 (16.5) | .802 | .658 | .877 | .570 |
| MACE | 111 (33) | 38 (32.2) | 38 (36.9) | .729 | .909 | .470 | .469 |
HF, heart failure; IAB, interatrial block; MACE, major adverse combined event.
Data are expressed as no. (%).
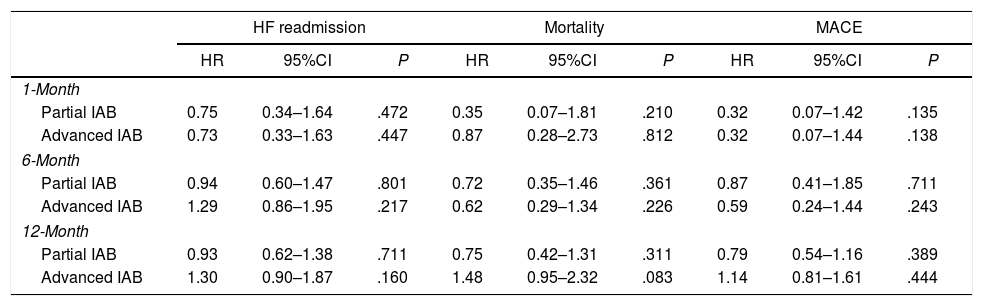
A Cox regression analysis adjusted by a set of ten relevant clinical variables (age, previous HF diagnosis, HF evolution in years, number of previous HF admissions, valvular HF etiology, left atrial diameter, N-terminal pro-B-type natriuretic peptide and GDF-15 values at discharge, heart rate at discharge, and left bundle branch block at discharge) showed no significant association of IAB pattern with the primary and secondary outcomes (Table 4). Fig. 2 of the supplementary data shows the absolute mean differences of the non-adjusted and adjusted model containing the ten relevant clinical variables.
Inverse probability of IPTWa analysis for risk of 1-, 6-, and 12-month HF admission, mortality and MACE.
| HF readmission | Mortality | MACE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| 1-Month | |||||||||
| Partial IAB | 0.75 | 0.34–1.64 | .472 | 0.35 | 0.07–1.81 | .210 | 0.32 | 0.07–1.42 | .135 |
| Advanced IAB | 0.73 | 0.33–1.63 | .447 | 0.87 | 0.28–2.73 | .812 | 0.32 | 0.07–1.44 | .138 |
| 6-Month | |||||||||
| Partial IAB | 0.94 | 0.60–1.47 | .801 | 0.72 | 0.35–1.46 | .361 | 0.87 | 0.41–1.85 | .711 |
| Advanced IAB | 1.29 | 0.86–1.95 | .217 | 0.62 | 0.29–1.34 | .226 | 0.59 | 0.24–1.44 | .243 |
| 12-Month | |||||||||
| Partial IAB | 0.93 | 0.62–1.38 | .711 | 0.75 | 0.42–1.31 | .311 | 0.79 | 0.54–1.16 | .389 |
| Advanced IAB | 1.30 | 0.90–1.87 | .160 | 1.48 | 0.95–2.32 | .083 | 1.14 | 0.81–1.61 | .444 |
CI, confidence interval; HF, heart failure; HF, heart failure; HR, hazard ratio; IAB, interatrial block; IPTW, inverse probability of treatment weighting; MACE, major adverse combined event;.
IPTW was done using the following independent variables: age, previous HF diagnosis, HF evolution in years, number of previous HF admissions, valvular HF etiology, left atrial diameter, N-terminal pro-B-type natriuretic peptide and GDF-15 values at discharge, heart rate at discharge, and left bundle branch block at discharge.
The presence of IAB is found in nearly 40% of patients hospitalized for HF, its behavior is dynamic throughout the clinical compensation, and is related with previous history of HF, valvular HF etiology, higher heart rate at admission, and bigger left atrial diameter. However, the persistence of this pattern at discharge does not imply a greater risk of readmission due to HF or death during the first year after discharge.
Prevalence, clinical profile, and dynamics of interatrial block in HF patientsThere are very few data on the prevalence of atrial conduction disorders in patients with HF. In a recent published series of 125 HF outpatients, Abdellah et al.10 reported a prevalence of IAB of 57.3%. Another study11 of 446 ambulatory patients with HF showed a prevalence of IAB of 44%. Both cohorts are outpatient single center series. As far as we know, our study is the first aimed to describe the prevalence of IAB in patients admitted for HF. For this purpose, we have employed a validated software and this could also explain the different prevalence in comparison with Abdellah et al. which used another method and caliper to measure P wave duration.
Our results draw the clinical profile of the patient with persistence of IAB at discharge. Both previous history of HF and a bigger left atrial diameter specially emphasize the role of IAB as an easy and non-invasive marker of disease progression. Moreover, our study provides the first data regarding dynamic changes of P wave during HF admission. It is remarkable that a substantial part of patients presenting advanced IAB at discharge were found in flutter or atrial fibrillation at admission (Fig. 2). From our point of view, this evolution reinforces the role of IAB as an intermediate step between healthy atria and atrial fibrillation, and as a risk predictor of atrial arrhythmias (Bayes’ syndrome).12,13 In addition, this dynamic behavior might reflect the decongestion process during HF hospitalization. Similar trend of changes have been described in other related clinical scenarios that might influence atrial pressures, like fluid overload before hemodialysis,14 selective atrial ischemia,15 or during exercise in patients with coronary artery disease.16
Prognostic implication of IABIAB have been recognized as predictors of atrial arrhythmias, stroke, and cognitive impairment.11,17 Furthermore, some studies have found an association of IAB with a higher cardiovascular readmissions and mortality,10 or a trend to a linear association between the degree of IAB and mortality in patients with previous myocardial infarction18 (HR, 1.61, 95%CI, 0.93–2.79) or even in general population19 (HR, 1.06; 95%CI, 1.00–1.13). On the contrary, Fernandes et al.20 found no association of advanced IAB with cardiovascular events. In the same line, our data do not support the association of any degree of IAB with a higher short- and mid-term mortality/readmission rate in patients discharged after a HF hospitalization. Given IAB may interpret as a surrogate of atrial enlargement, it is possible that the potential effect of IAB on prognosis is diluted against other powerful prognostic markers of HF, such as natriuretic peptides, mitral regurgitation or renal impairment function.
LimitationsThis study is a large multicenter nationwide observational registry, including a 98% of Caucasians, thus our data might not be fully applicable to other ethnicities or countries. Moreover, the size of the study sample did not allow analyzing the prognostic role of IAB in subgroups of clinical interest. A non-negligible number of patients (688 out of 1831, 37%) were excluded due to absence of available 12-lead ECG both on admission or discharge. In relation with this, Table 1 of the supplementary data shows the differences regarding to the clinical characteristics between the study population and excluded patients. In addition, the C-statistic index of each IPTW model to assess the relationship of type of IAB with the primary and secondary outcomes were modest (0.627 for “Partial IAB” and 0.727 for “Advanced IAB”, respectively), so our results should be interpreted cautiously. Given that the REDINSCOR II Registry was designed in 2013, HF was diagnosed in accordance with current HF guidelines in that time period. Lastly, the follow-up only covers 1 year after discharge, so it may not be enough to test a significant effect of the IAB on the prognosis. New studies with longer follow-up are warranted to confirm the potential prognostic impact of IAB in HF.
ConclusionsIAB is frequent in patients in sinus rhythm admitted for HF, related with previous history of HF and bigger left atria, with dynamic changes throughout hospitalization. However, the persistence of IAB at discharge does not imply a greater short- or mid-term risk of readmission HF or death.
- -
IAB is correlated with atrial enlargement and dysfunction, and is strongly associated with atrial tachyarrhythmia, particularly atrial fibrillation, as well as embolic stroke.
- -
A careful ECG analysis is useful in order to obtain essential information for the management and prognostic evaluation of patients with HF.
- -
Impairment of interatrial conduction may occur in patients with HF due to structural anomaly and/or increased atrial filling pressure.
- -
IAB is found in 40% of patients in sinus rhythm discharged after a HF hospitalization.
- -
The independent factors related with IAB were previous history of HF, valvular HF etiology, heart rate at admission, and left atrial diameter.
- -
This ECG pattern at discharge does not imply a greater risk of readmission or death during the first year.
This study was supported by grants from Redes Temáticas de Investigación Cooperativa en Salud del Instituto de Salud Carlos III (REDINSCOR), Madrid, Spain, grant no. RD06-0003-0000 and Red de Investigación Cardiovascular del Instituto de Salud Carlos III (RIC), Madrid, Spain, grant no. RD12/0042/0002.
Conflicts of interestThe authors report no conflicts of interest.
Abbreviations: ECG: electrocardiogram; HF: heart failure; IAB: interatrial block.