Conduction disturbances (CD) after transcatheter aortic valve implantation (TAVI) increase the risk of permanent pacemaker (PPM) implantation and often preclude early-discharge (ED). The aim of this study was to evaluate the performance of the 2019 consensus algorithm on the management of conduction disturbances post-TAVI in a real-world cohort.
MethodsRetrospective analysis of patients who underwent transfemoral TAVI between 2016 and 2018. Patients with prior PPM were excluded. The algorithm classified patients as ED or non-ED candidates (due to a higher risk for high-degree atrioventricular block or because they were eligible for PPM). ED was defined as discharge at day-3 post-TAVI. Primary endpoint was the 30-day rate of PPM implantation and secondary endpoints included 6-month pacing rate, 1-year rate of syncope and all-cause mortality and the composite outcome of both, regarding ED eligibility.
ResultsA total of 220 patients were included. The algorithm classified 66% of the patients as ED candidates. The 30-day rate of PPM implantation was 3.6% for ED candidates (vs 62.5% for non-ED candidates; P<.001). No differences were found in the secondary endpoints between ED and non-ED candidates. The algorithm identified 16% of the patients as eligible for PPM of whom 97.1% underwent PPM implantation; 18% of the patients were classified as at higher risk of high-degree atrioventricular block.
ConclusionsThe 2019 algorithm allowed to safely ED a significant number of patients with conduction disturbances post-TAVI and to identify patients with a clear indication for PPM implantation. However, in high-risk patients, the algorithm does not provide a guiding strategy.
Los trastornos de la conducción tras el implante percutáneo de válvula aórtica (TAVI) incrementan el riesgo de necesidad de implante de marcapasos permanente (MP) y frecuentemente evitan un alta precoz (AP). El objetivo de nuestro estudio fue evaluar el rendimiento del algoritmo de consenso de 2019 en el tratamiento de los trastornos de la conducción post-TAVI en una cohorte real de pacientes.
MétodosAnálisis retrospectivo de pacientes que se sometieron a un TAVI transfemoral entre 2016 y 2018. Se excluyó a los pacientes que previamente habían sido portadores de MP. Se clasificó a los pacientes como candidatos a AP o no candidatos (en función del riesgo de bloqueo auriculoventricular de alto grado o la necesidad de implante de MP) según el algoritmo. Se definió el AP como el alta al tercer día post-TAVI. El objetivo principal fue la tasa a 30 días de implante de MP y los secundarios fueron la tasa a 6 meses de estimulación por MP, la tasa a un año de síncope y la mortalidad por cualquier causa.
ResultadosSe incluyó a un total de 220 pacientes. El 66% de los pacientes se consideró candidato a AP según el algoritmo. La tasa de implante de MP a 30 días fue de 3,6% para los candidatos a AP frente a 62,5% en los no candidatos (p<0,001). No se observaron diferencias en los objetivos secundarios entre los candidatos y no candidatos a AP. El 16% de los pacientes fueron candidatos a implante de MP, de los cuales el 97,1% se sometieron a dicho implante; el 18% de los pacientes se clasificó como en alto riesgo de bloqueo auriculoventricular de alto grado.
ConclusionesEl algoritmo de 2019 permite un AP segura en una parte significativa de los pacientes con trastornos de la conducción post-TAVI, así como la identificación de aquellos pacientes con indicación de implante de MP. Sin embargo, en pacientes de alto riesgo no es de ayuda en el tratamiento.
Transcatheter aortic valve implantation (TAVI) has become a well-established treatment of severe aortic stenosis, particularly in older patients with high surgical risk.1 Increased evidence from recent randomized clinical trials have demonstrated that the favourable clinical outcomes and safety profile of TAVI also extend to intermediate2 and low-risk patients.3,4
The widespread use of TAVI, particularly in low-risk and younger patients, raised relevant questions, regarding long-term valve durability and the rate of permanent pacemaker (PPM) implantation. Also, the management of conduction disturbances (CD) after the procedure, the criteria for PPM implantation, and the timing for discharge became increasingly challenging.
Advances in TAVI technology, combined with an increasing number of procedures and operator experience, better patient selection and pre-procedural planning, have led to a reduction in periprocedural complications and death. However, this trend was not translated into a reduction of CD after TAVI, which remain one of the main complications of the procedure,5 associated with high rates of PPM implantation,6 ranging between 2% and 51%.7
Some studies have tried to identify electrocardiographic predictors of high-degree atrioventricular block8,9 (HAVB) and the need for PPM implantation10,11 after TAVI, in order to identify patients who could be early and safely discharged.10 The number of patients being discharged on the same day of the procedure12 and at 24–48h is increasing13; the multicentre European FAST-TAVI trial14 showed that a standardized criteria defined for early-discharge (ED) allowed a safe and timely discharge (≤3 days) of patients who underwent transfemoral balloon-expandable valve. However, the discharge criteria excluded no untreated major arrhythmias. In most studies, patients with baseline bundle brunch block and who develop CD post-TAVI were excluded.
Until recently, no standardized clinical pathway has been conducted to guide the management of CD after TAVI, in order to identify patients who could be safely discharged, those with higher risk for CD and those who have a recommendation for PPM implantation. A recent interdisciplinary expert consensus document has been published, providing guidelines for the management of CD after TAVI.15
The aim of this study was to apply and to evaluate the performance of the adapted algorithm from the 2019 consensus document,15 in identifying patients who could be discharged early and safely or who would have an indication for PPM, in a real-world cohort.
MethodsStudy setting and sampleAll patients older than 18 years old with severe symptomatic aortic stenosis, who underwent TAVI between January 2016 and December 2018 in a tertiary hospital, were analyzed retrospectively. Patients with pre-existing PPM, nontransfemoral approach, valve-in-valve procedures and intraprocedural death were excluded.
The local ethics review board approved this study and written informed consent was waived due to the study's retrospective nature. The study was conducted following the declaration of Helsinki.
TAVI procedureAll patients proposed for TAVI were evaluated by a multidisciplinary heart team and the eligibility for TAVI procedure was decided by consensus. Pre-procedure cardiac and iliofemoral access computed tomography angiography were performed in all patients. The type of prosthesis (self-expandable vs balloon-expandable) was chosen by the heart team.
Overall, patients were electively admitted to the hospital on the day of the procedure, and all underwent transfemoral TAVI in a cardiac catheterization laboratory. The procedures were performed under local anaesthesia and minimal sedation, with continuous haemodynamic and electrocardiographic monitoring. Femoral artery access was obtained for all patients, under fluoroscopy or ultrasound guidance. Femoral punctures were routinely preclosed using 2 Perclose ProGlide devices (Abbott Vascular, United States) or, more recently, one Perclose and one Angio-Seal 8-Fr (Terumo, Japan).
Pacing method was at the operator's discretion. In most cases, the temporary pacemaker was removed in the catheterization laboratory if considered suitable by the operator.
After the procedure, all patients were admitted at the cardiac intensive care unit.
Indications for permanent pacemaker implantation and timing of dischargeThe indication for PPM implantation and timing of discharge were at the discretion of the treating physician and in accordance with the pacing and electrophysiology laboratory team. No electrophysiological studies were conducted.
Data collectionClinical, procedural, electrocardiographic and follow-up data were collected through hospital records. Patients were followed by regular outpatient visits at our centre or the referring hospital; decisions regarding the frequency of visits were at the treating physician's discretion.
Variables definitions are presented in supplementary data.
Electrocardiogram analysisStandard 12-lead electrocardiograms (ECG) were collected at baseline, immediately after the procedure, daily until day 3 post-TAVI and at discharge. Electrocardiograms were recorded using standard calibration (10mm=1mV, 25mm/s). The ECGs were retrospectively analyzed by the same investigator and the following parameters were assessed: rhythm, PR interval, presence, and type of atrioventricular (AV) block, QRS interval, presence of bundle-branch block, left anterior fascicular block, left posterior fascicular block or nonspecific intraventricular conduction disturbance with QRS interval≥120ms. All these analyses were performed according to the recommendations for the standardization and interpretation of ECG,16 and based on the definitions of CD after TAVI, proposed by the 2019 consensus document15 (supplementary data). Additionally, continuous telemetry monitoring was recorded during the procedure and during all hospital stay.
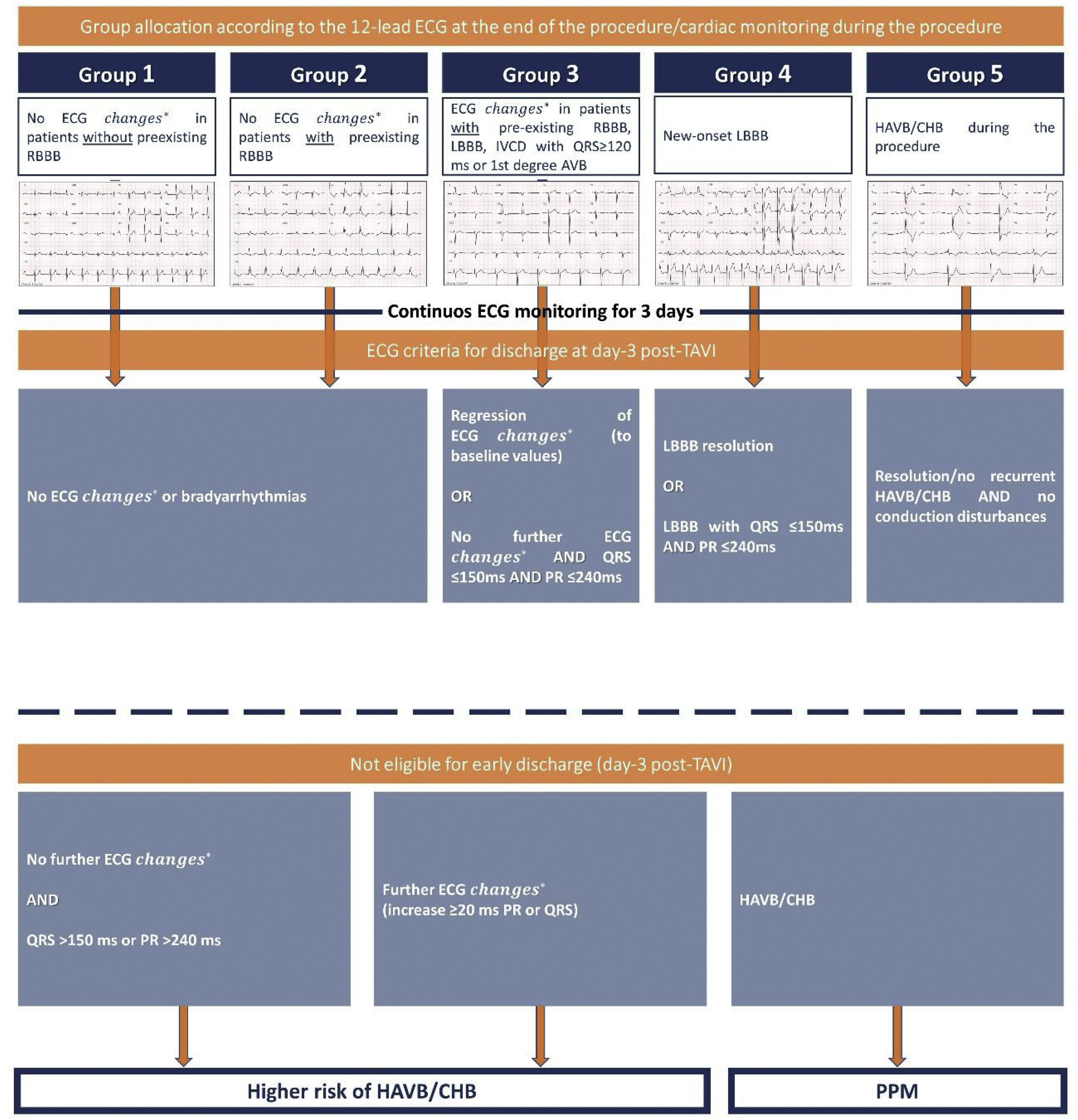
Adapted algorithm from the 2019 Scientific Expert Panel consensus documentAccording to the 2019 Scientific Expert Panel consensus document,15 and after analysing the information obtained from the baseline 12-lead ECG, continuous telemetry monitoring during the procedure and 12-lead ECG immediately after the procedure, patients were retrospectively classified in 5 groups (Fig. 1): group 1, no ECG changes in patients without pre-procedure right bundle brunch block (RBBB); group 2, no ECG changes in patients with preexisting RBBB; group 3, ECG changes in patients with preexisting CD; group 4, new-onset left bundle brunch block (LBBB) and group 5, HAVB or complete heart block (CHB) during the periprocedural period.
Group allocation and criteria for early discharge according to the conduction disturbances following TAVI. Adapted algorithm from the 2019 Management of Conduction Disturbances Associated With Transcatheter Aortic Valve Replacement. Modified with permission from Rodés Cabau et al.15 AVB, atrioventricular block; CHB, complete heart block; ECG, electrocardiogram; HAVB, high-degree atrioventricular block; IVCD, intraventricular conduction delay; LBBB, left bundle brunch block; RBBB, right bundle brunch block. *1st degree AVB, RBBB, LBBB or QRS≥120ms with IVCD.
The proposed algorithm15 (Fig. 1) was adapted and retrospectively applied in our cohort to identify which patients could have been eligible for ED with no further measures, and which patients were not suitable for ED, due to a higher risk for HAVB/CHB or because they were candidates for PPM implantation (for more details regarding ED criteria, refer to supplementary data).
Early-discharge was defined as discharge at day-3 after the procedure.
Primary and secondary endpointsThe primary endpoint was defined as the rate of PPM implantation within 30 days after TAVI regarding eligibility for ED. Secondary endpoints included 6-month atrial and/or ventricular pacing rates, 1-year rate of syncope and all-cause mortality and the composite outcome of both, in relation with ED eligibility.
Statistical analysisDescriptive statistics for categorical variables were summarized as absolute and relative frequencies and continuous variables were expressed as median and interquartile range [IQR].
Patients’ characteristics were compared between patients with and without ED using χ2 test or Fisher's exact test, as appropriate; or Student t test or Mann–Whitney U test, in case of non-Gaussian distribution using the Kolmogorov–Smirnov test.
Statistical significance was set at a 2-tailed probability level of <0.05. All statistical analysis was performed using Statistical Package for Social Sciences (SPSS) software version 23.0 (IBM, Armonk, USA).
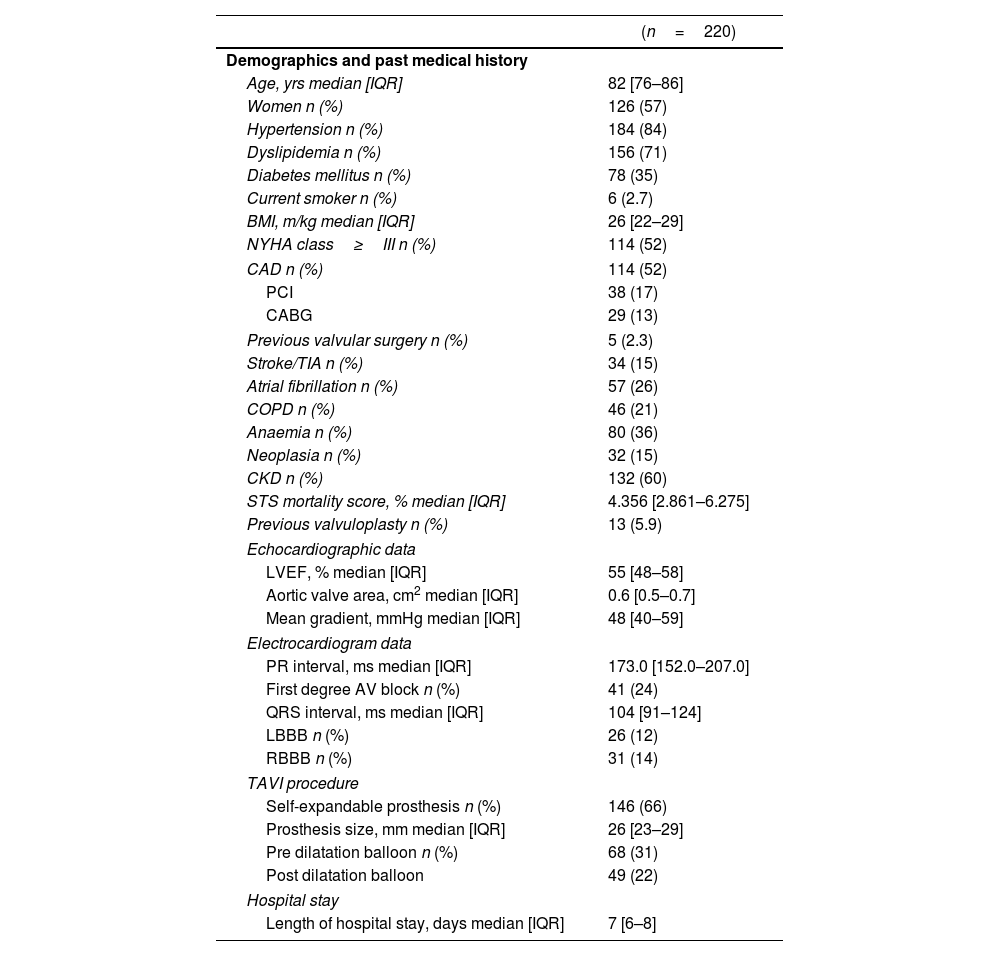
ResultsStudy sampleA total of 220 patients underwent transfemoral TAVI between January 2016 and December 2018. The median age was 82 years [76–86] and 126 patients (57%) were women. Median Society of Thoracic Surgery mortality score was 4.356% [2.861–6.275] and a self-expandable prosthesis was used in 146 patients (66%). Baseline demographic, clinical and procedural characteristics of the study sample are summarized in Table 1.
Baseline clinical and procedural characteristics of the population.
| (n=220) | |
|---|---|
| Demographics and past medical history | |
| Age, yrs median [IQR] | 82 [76–86] |
| Women n (%) | 126 (57) |
| Hypertension n (%) | 184 (84) |
| Dyslipidemia n (%) | 156 (71) |
| Diabetes mellitus n (%) | 78 (35) |
| Current smoker n (%) | 6 (2.7) |
| BMI, m/kg median [IQR] | 26 [22–29] |
| NYHA class≥III n (%) | 114 (52) |
| CAD n (%) | 114 (52) |
| PCI | 38 (17) |
| CABG | 29 (13) |
| Previous valvular surgery n (%) | 5 (2.3) |
| Stroke/TIA n (%) | 34 (15) |
| Atrial fibrillation n (%) | 57 (26) |
| COPD n (%) | 46 (21) |
| Anaemia n (%) | 80 (36) |
| Neoplasia n (%) | 32 (15) |
| CKD n (%) | 132 (60) |
| STS mortality score, % median [IQR] | 4.356 [2.861–6.275] |
| Previous valvuloplasty n (%) | 13 (5.9) |
| Echocardiographic data | |
| LVEF, % median [IQR] | 55 [48–58] |
| Aortic valve area, cm2 median [IQR] | 0.6 [0.5–0.7] |
| Mean gradient, mmHg median [IQR] | 48 [40–59] |
| Electrocardiogram data | |
| PR interval, ms median [IQR] | 173.0 [152.0–207.0] |
| First degree AV block n (%) | 41 (24) |
| QRS interval, ms median [IQR] | 104 [91–124] |
| LBBB n (%) | 26 (12) |
| RBBB n (%) | 31 (14) |
| TAVI procedure | |
| Self-expandable prosthesis n (%) | 146 (66) |
| Prosthesis size, mm median [IQR] | 26 [23–29] |
| Pre dilatation balloon n (%) | 68 (31) |
| Post dilatation balloon | 49 (22) |
| Hospital stay | |
| Length of hospital stay, days median [IQR] | 7 [6–8] |
AF, atrial fibrillation; AV, atrioventricular; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LBBB, left bundle brunch block; LVEF, left ventricle ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RBBB, right bundle brunch block; STS, Society of Thoracic Surgery; TAVI, transcatheter aortic valve implantation; TIA, transient ischaemic attack.
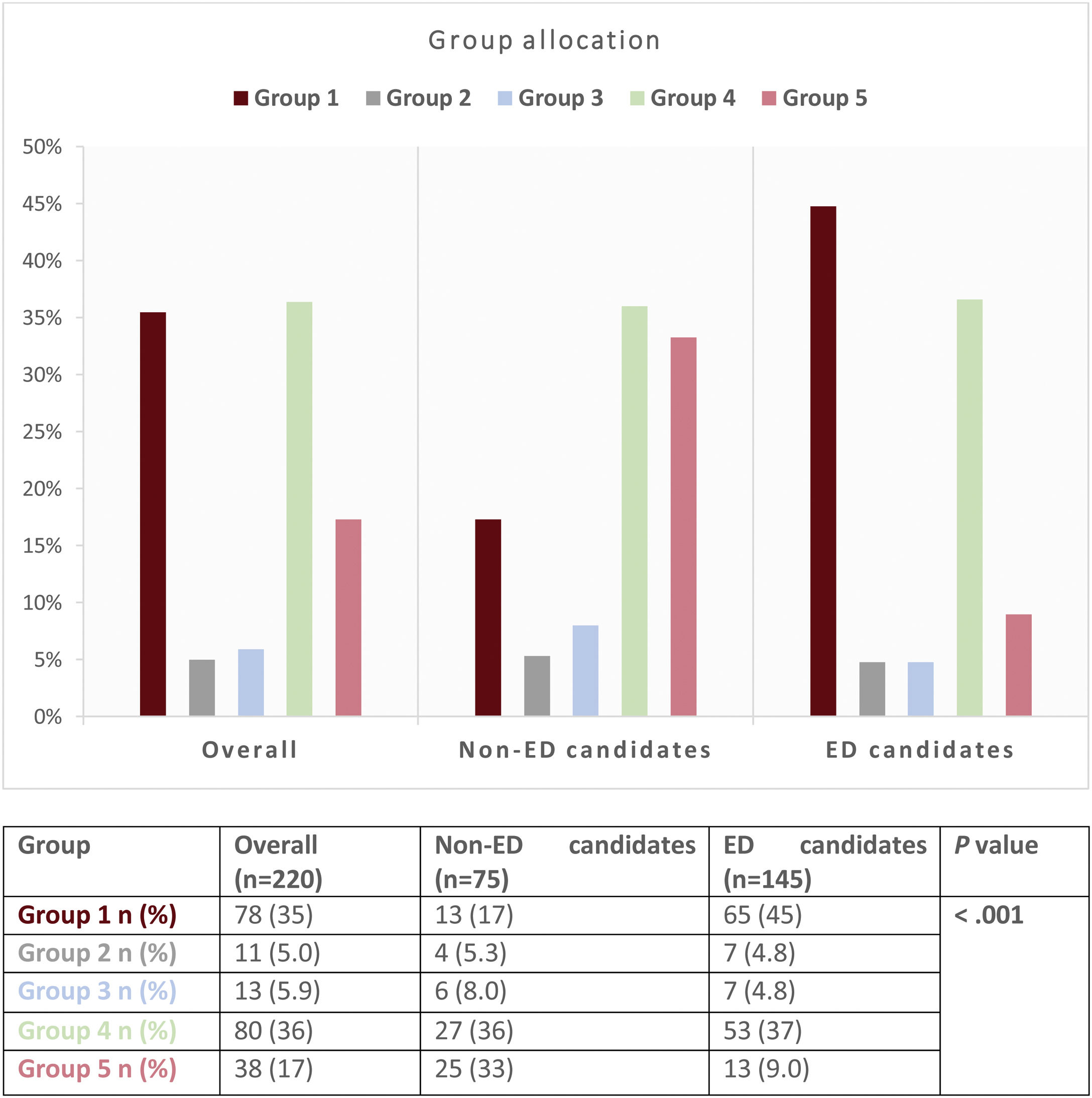
Patients’ allocation to the 5 pre-defined groups is shown in Fig. 2. Seventy-eight patients (35%) were categorized in group 1, 11 patients (5.0%) in group 2, 13 patients (5.9%) in group 3, 80 patients (36%) in group 4 and 38 patients (17%) in group 5.
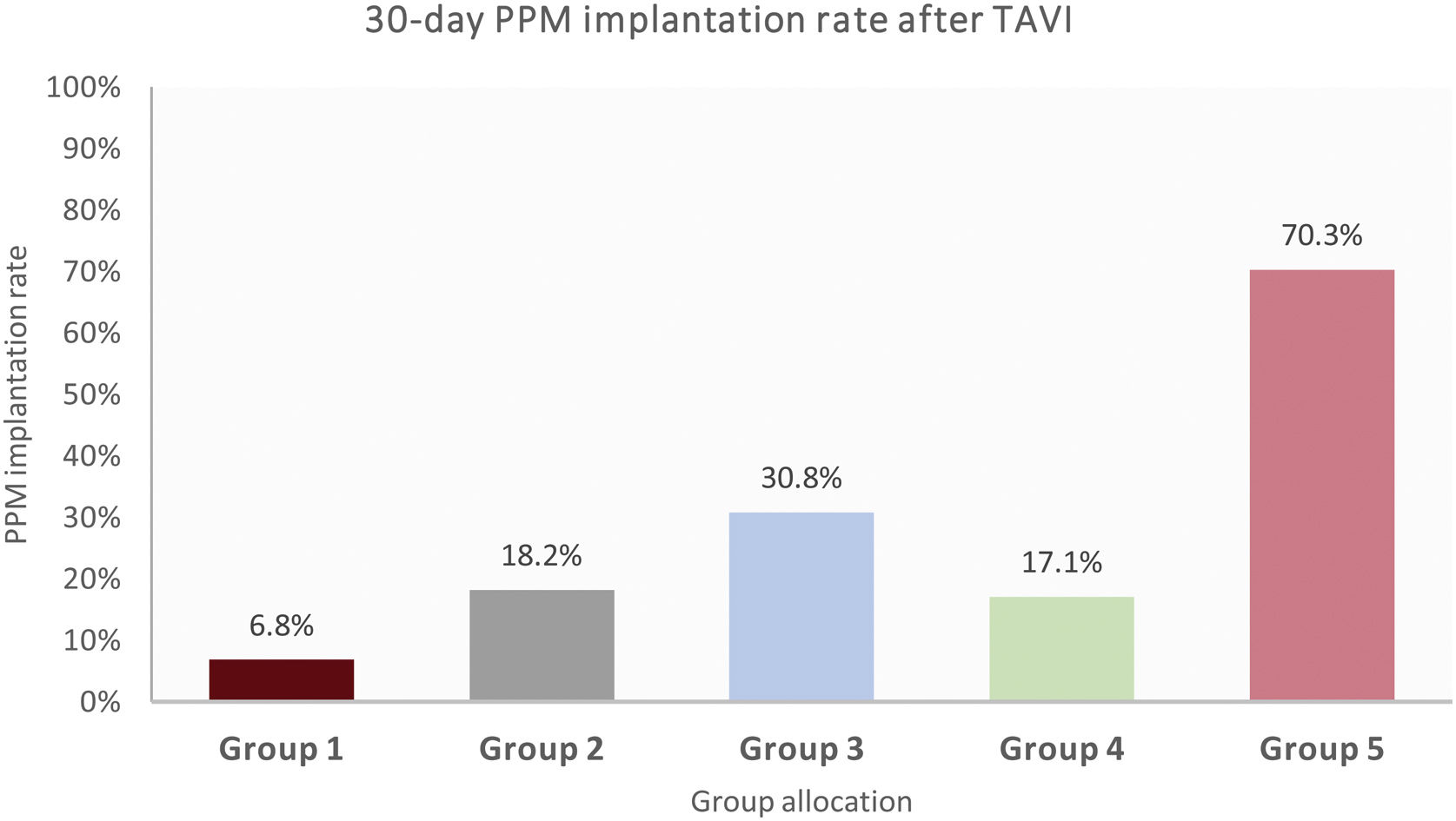
The 30-day PPM implantation rate as a function of group allocation is shown in Fig. 3. Group 5 showed the highest PPM rates (70.3%), followed by group 3 (30.8%).
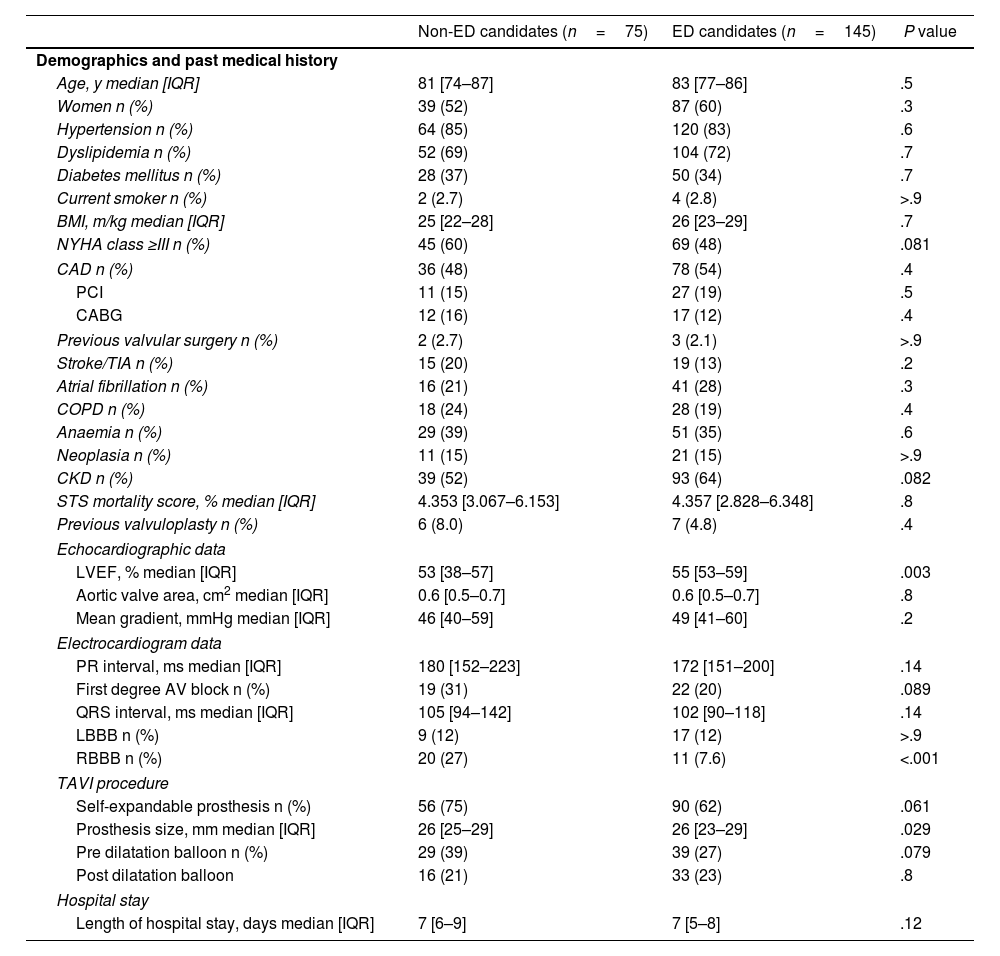
Early-discharge eligibilityOne hundred and forty-five patients (65.9%) were considered ED candidates and 75 patients (34.1%) non-ED candidates. Baseline demographic, clinical and procedural characteristics comparing ED with non-ED candidates are present in Table 2. Early-discharge candidates presented a significant higher median left ventricle ejection fraction (55% [IQR 53–59] vs 53% [IQR 38–57]; P=.003) and lower median prosthesis size (26mm [23–29] vs 26mm [25–29]; P=.029) than non-ED candidates. There were no significant differences between ED and non-ED candidates regarding the type of valve implanted (self-expandable vs balloon-expandable), although there was a greater tendency for higher rates of ED in patients who underwent balloon-expandable valve implantation (74% vs 62%; P=.061) (Fig. 1 of the supplementary data).
Baseline clinical and procedural characteristics regarding early-discharge eligibility.
| Non-ED candidates (n=75) | ED candidates (n=145) | P value | |
|---|---|---|---|
| Demographics and past medical history | |||
| Age, y median [IQR] | 81 [74–87] | 83 [77–86] | .5 |
| Women n (%) | 39 (52) | 87 (60) | .3 |
| Hypertension n (%) | 64 (85) | 120 (83) | .6 |
| Dyslipidemia n (%) | 52 (69) | 104 (72) | .7 |
| Diabetes mellitus n (%) | 28 (37) | 50 (34) | .7 |
| Current smoker n (%) | 2 (2.7) | 4 (2.8) | >.9 |
| BMI, m/kg median [IQR] | 25 [22–28] | 26 [23–29] | .7 |
| NYHA class ≥III n (%) | 45 (60) | 69 (48) | .081 |
| CAD n (%) | 36 (48) | 78 (54) | .4 |
| PCI | 11 (15) | 27 (19) | .5 |
| CABG | 12 (16) | 17 (12) | .4 |
| Previous valvular surgery n (%) | 2 (2.7) | 3 (2.1) | >.9 |
| Stroke/TIA n (%) | 15 (20) | 19 (13) | .2 |
| Atrial fibrillation n (%) | 16 (21) | 41 (28) | .3 |
| COPD n (%) | 18 (24) | 28 (19) | .4 |
| Anaemia n (%) | 29 (39) | 51 (35) | .6 |
| Neoplasia n (%) | 11 (15) | 21 (15) | >.9 |
| CKD n (%) | 39 (52) | 93 (64) | .082 |
| STS mortality score, % median [IQR] | 4.353 [3.067–6.153] | 4.357 [2.828–6.348] | .8 |
| Previous valvuloplasty n (%) | 6 (8.0) | 7 (4.8) | .4 |
| Echocardiographic data | |||
| LVEF, % median [IQR] | 53 [38–57] | 55 [53–59] | .003 |
| Aortic valve area, cm2 median [IQR] | 0.6 [0.5–0.7] | 0.6 [0.5–0.7] | .8 |
| Mean gradient, mmHg median [IQR] | 46 [40–59] | 49 [41–60] | .2 |
| Electrocardiogram data | |||
| PR interval, ms median [IQR] | 180 [152–223] | 172 [151–200] | .14 |
| First degree AV block n (%) | 19 (31) | 22 (20) | .089 |
| QRS interval, ms median [IQR] | 105 [94–142] | 102 [90–118] | .14 |
| LBBB n (%) | 9 (12) | 17 (12) | >.9 |
| RBBB n (%) | 20 (27) | 11 (7.6) | <.001 |
| TAVI procedure | |||
| Self-expandable prosthesis n (%) | 56 (75) | 90 (62) | .061 |
| Prosthesis size, mm median [IQR] | 26 [25–29] | 26 [23–29] | .029 |
| Pre dilatation balloon n (%) | 29 (39) | 39 (27) | .079 |
| Post dilatation balloon | 16 (21) | 33 (23) | .8 |
| Hospital stay | |||
| Length of hospital stay, days median [IQR] | 7 [6–9] | 7 [5–8] | .12 |
AF, atrial fibrillation; AV, atrioventricular; BMI, Body Mass Index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ED, early-discharge; LBBB, left bundle brunch block; LVEF, left ventricle ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RBBB, right bundle brunch block; STS, Society of Thoracic Surgery; TAVI, transcatheter aortic valve implantation; TIA, transient ischaemic attack.
Baseline median PR (172ms [151–200] vs 180ms [152–223]; P=.14) and QRS interval (102ms [90–118] vs 105ms [94–142]; P=.14) were similar between ED and non-ED candidates, as well as the prevalence of pre-existing LBBB (12% in both groups; P>.9). However, ED candidates had a significantly lower prevalence of RBBB prior intervention (7.6% vs 27%; P<.001).
The association between ED eligibility and group assignment according to the consensus document is shown in Fig. 2. Early-discharge candidates were classified in group 1 significantly more often than non-ED candidates (45% vs 17%) and less often in group 5 (9.0% vs 33%) (overall P value<.001).
The overall median length of hospital stay until discharge was 7 days [IQR 6–8]. Only 7 patients were effectively discharged within 3 days after TAVI. Of those, 3 patients were prospectively classified as ED candidates and 4 patients as non-ED candidates. The latter underwent PPM implantation during the first days of hospital stay, allowing discharge within 3 days post-TAVI. There were no significant differences in median length of hospital stay between patients classified as ED (7 days [IQR 5–8]) and non-ED candidates (7 days [6–9.5]); P=.116.
Primary endpointThe 30-day rate of PPM implantation for ED candidates was 3.6% and for non-ED candidates was 62.5% (P<.001), all implanted during index hospital stay. The median time until PPM implantation for ED candidates was 6.0 days [5.0–9.5] and 2.0 days [1.0–5.0] for non-ED candidates (P=.011).
Early-discharge candidates who underwent PPM implantationFive patients eligible for ED underwent PPM implantation within 30 days after TAVI: one patient was assigned to each of groups 1, 3 and 4, and 2 patients to group 5.
The majority of these patients (3 patients) underwent PPM implantation due to sinus node dysfunction. Of those, none presented syncope or haemodynamic instability. The 6-month rate of atrial and ventricular pacing was: a) 47.5% and 11.3%, b) 88% and 93%, and c) 89.6% and <1%, respectively. The remaining 2 patients underwent PPM implantation due to CHB (at day 5 and 6 after TAVI); one presented syncope and required temporary transvenous pacemaker. The 6-month rate of ventricular pacing was 10% and 2%, respectively.
None of these patients showed pacemaker dependency at 6-month follow-up.
Secondary endpointThe 6-month rate of atrial pacing was higher in ED candidates (47.5% [1.0–88.8] vs 14.5% [3.4–39.5]; P=.546) and the rate of ventricular pacing was lower in ED candidates (10.0% [1.0–93.0] vs 85.5% [8.5–99.0]; P=.146), although the difference was not statistically significant (P>.05). No differences were reported regarding 1-year mortality (4.9% vs 6.9%; P=.750), 1-year prevalence of syncope (2.9% vs 4.7%; P=.820) and the composite outcome of 1-year mortality or syncope (7.9% vs 11.8%; P=.510), between ED and non-ED candidates.
Non-early-discharge candidatesSeventy-five patients (34.1%) were not eligible for ED: 35 patients (15.9%) were classified as candidates for PPM implantation and 40 patients (18.2%) as at higher risk for HAVB/CHB.
Candidates for PPM implantation were more often assigned to group 5 (68.6% vs 2.5%) and less to group 4 (11.4% vs 57.5%) than patients at higher risk for HAVB/CHB (Table 3).
Group allocation for non-early-discharge candidates.
| Group allocation | PPM candidates (n=35) | High risk for HAVB/CHB (n=40) |
|---|---|---|
| Group 1 n (%) | 3 (8.6) | 10 (25.0) |
| Group 2 n (%) | 3 (8.6) | 1 (2.5) |
| Group 3 n (%) | 1 (2.9) | 5 (12.5) |
| Group 4 n (%) | 4 (11.4) | 23 (57.5) |
| Group 5 n (%) | 24 (68.6) | 1 (2.5) |
Group 1, no ECG changes in patients without pre-procedure RBBB; group 2, no ECG changes in patients with preexisting RBBB; group 3, ECG changes in patients with preexisting CD; group 4, new-onset LBBB and group 5, HAVB/CHB during the periprocedural period. CD, conduction disturbances; CHB, complete heart block; ECG, electrocardiography; ED, early-discharge; HAVB, high-degree atrioventricular block; LBBB, left bundle brunch block; PPM, permanent pacemaker implantation; RBBB, right bundle brunch block.
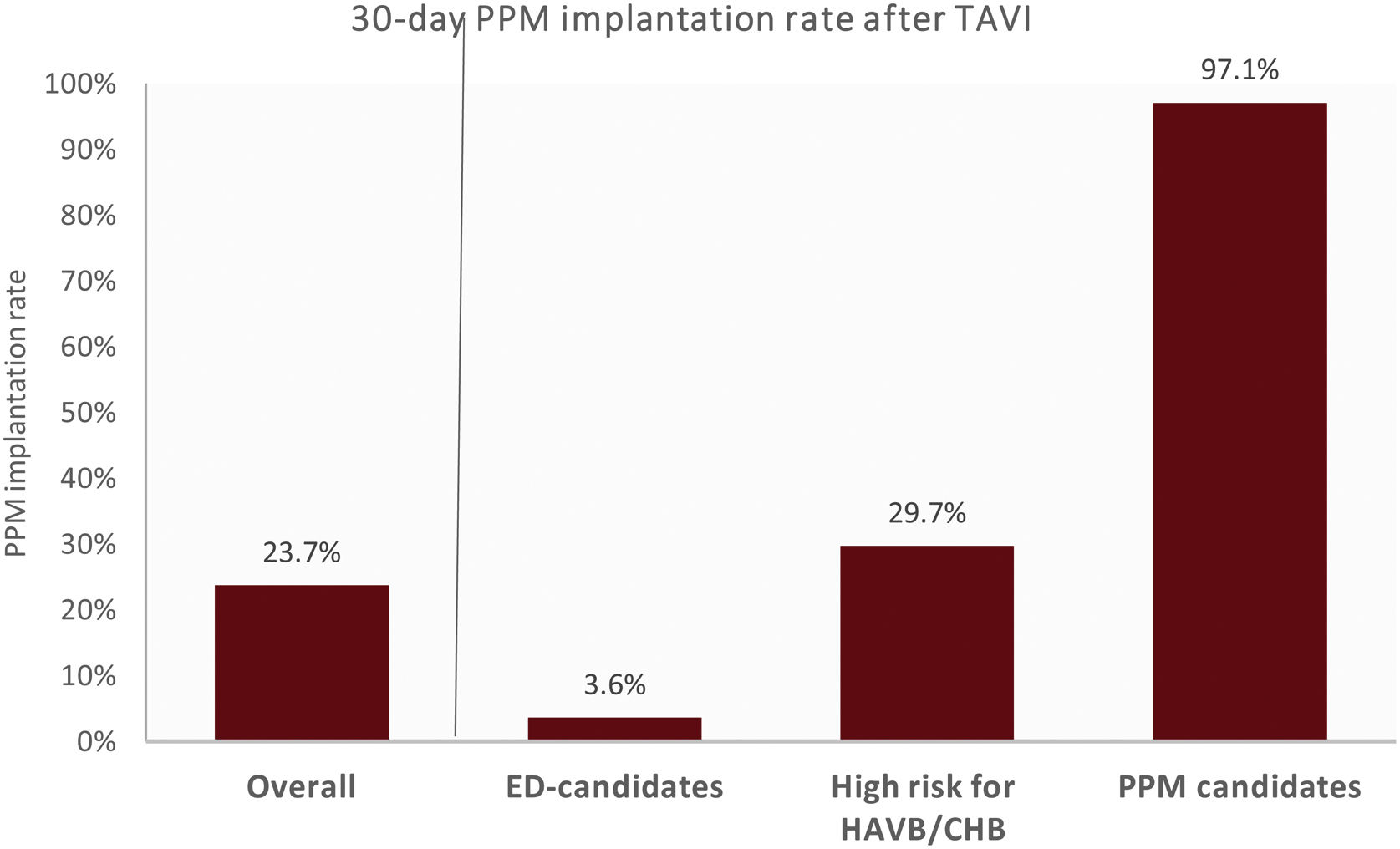
According to the suggested algorithm, 97.1% of the patients classified as candidates for PPM implantation and 29.7% of the patients classified as at higher risk for HAVB/CHB effectively underwent PPM implantation within 30 days after TAVI (Fig. 4). PPM was implanted on average at day one and six after TAVI, for PPM implantation candidates and for patients at higher risk for HAVB/CHB, respectively. The 6-month median atrial and ventricular pacing rates was 9.9% [1.4–21.5] and 97.5% [53.3–99.0] for PPM implantation candidates and 37% [22.3–76.4] and 3.4% [1.0–36.1] for patients at higher risk for HAVB/CHB, respectively.
30-Day permanent pacemaker implantation rate after transcatheter aortic valve implantation, according to the algorithm recommendations of the 2019 consensus document. CHB, complete heart block; ED, early-discharge; HAVB, high-degree atrioventricular block; PPM, permanent pacemaker; TAVI, transcatheter aortic valve implantation.
There were no significant differences between the type of valve implanted (self-expandable vs balloon-expandable) regarding group allocation and according to the algorithm recommendations of the 2019 consensus document (Figs. 1–3 of the supplementary data). However, patients with balloon-expandable valves were more frequently allocated to Group 1 (46% vs 30%) and less frequently to Group 4 (30% vs 40%) and there was a lower tendency for them to be classified as higher risk for HAVB/CHB (10% vs 23%; P=.053). There were no significant differences in the 30-day rate of PPM implantation between the type of valve implanted (P=.296).
DiscussionIn this real-world study, the implementation of an adapted algorithm on Management of Conduction Disturbances associated with TAVI15 classified a significant number of patients (66%) as eligible for ED; only 3.6% of these underwent PPM implantation within 30-days after the procedure; no differences were found regarding 1-year incidence of mortality, syncope, and the composite outcome of both between ED and non-ED candidates. Sixteen percent of the patients were classified as candidates for PPM implantation and almost all of them (97.1%) effectively underwent PPM implantation. Lastly, 18% of the patients were classified as high risk for HAVB/CHB.
Characteristics of the patients eligible for early-dischargeThe high prevalence of CD after TAVI, along with the high rate of PPM implantation, and the uncertainty regarding the timing of discharge, render the management of patients who develop CD post-TAVI a challenge. A recent multidisciplinary expert consensus document has been published to provide a standardize pathway for the management of CD after TAVI.15
In our cohort, the adapted algorithm identified a significant number of patients (66%) as eligible for discharge within 3 days after TAVI. In those patients, the prevalence of pre-existing LBBB and RBBB was 12% and 7.6%, respectively. Almost half of the ED candidates (45%) were classified in group 1 (no ECG changes in patients without RBBB pre-procedure). Malebranche et al.17 validated the same algorithm in a large cohort and also found that most of the patients (73%) were eligible for ED, with similar prevalence of LBBB and RBBB as in our study (11% and 6%, respectively). However, the incidence of patients assigned to group 1 was higher than in our cohort (59% vs 45%), which may explain the increased prevalence of patients suitable for ED than in our study.
We found that patients eligible for ED had a lower prevalence of pre-existing RBBB than non-ED candidates (7.6% vs 27%), without differences in the prevalence of baseline LBBB.
Although in the proposed algorithm, baseline RBBB doesn’t preclude an ED strategy, the clinical management of these patients is still a matter of great debate. Pre-existing RBBB seems to be the strongest and most consistent risk factor for PPM implantation after TAVI.11,18 Auffret et al.19 found that patients with baseline RBBB who underwent TAVI and were discharged without PPM during the index hospitalization, were at higher risk of HAVB and/or sudden cardiac death during follow-up. Another study found that all-cause mortality and cardiovascular mortality was higher in patients with pre-existing RBBB.20 The higher incidence of CD in patients with baseline RBBB make them less suitable for an ED strategy, which is consistent with our findings. However, in our cohort, non-ED candidates didn’t show an increase in 1-year mortality, despite having a higher prevalence of baseline RBBB. This could be explained because almost 65% of non-ED patients underwent PPM implantation, protecting them from HAVB and making them less vulnerable to sudden cardiac death.
Type of valve implanted (self-expandable vs balloon-expandable)Despite no significant differences were found regarding eligibility for ED between the type of valve implanted (self-expandable vs balloon-expandable), there was a greater tendency for higher rates of ED in patients who underwent balloon-expandable valve implantation. Patients with balloon-expandable valves were more frequently allocated to Group 1 and less frequently to Group 4. Additionally, there was a lower tendency for them to be classified as higher risk for HAVB/CHB. It is well established that self-expandable valve implantation is a major predictor of CD after TAVI,21 which is in line with the findings of our study. These differences may explain the higher number of patients with balloon-expandable valves classified as suitable for ED in our cohort.
However, due to the relatively small sample size and because it is a secondary analysis in a subgroup, this may have contributed to not finding significant differences between the type of valve implanted.
Safety and efficacy of an early-discharge algorithmThe 30-day rate of PPM implantation for ED candidates was 3.6%, similar to the rate reported by Malebranche et al.17 (2.7%). In our cohort, ED candidates underwent PPM implantation on average 6 days after TAVI and the majority had a PPM due to bradycardia–tachycardia syndrome, to enable the initiation and titration of pharmacological treatment for atrial fibrillation and to protect from the prolonged sinus pause following the termination of tachycardia, which translated into high rates of atrial pacing during follow-up. In general, pacing for sinus node dysfunction does not seem to affect survival,22 as opposed to pacing for HAVB/CHB.
Also, no significant differences were observed regarding 1-year mortality, syncope, or the composite outcome of both, between patients eligible for ED and those who were not.
When applied to the real-world practice, and in line with the latest evidence,17 the proposed algorithm appears to identify low-risk patients, who could have been ED. Also, it presented a high predictive negative value for subsequent need for PPM implantation, allowing an accurate recognition of patients suitable for ED and that will not benefit from PPM implantation.
However, since the results of this study are based on a retrospective analysis and as we did not discharged patients who would be candidates for ED, we may not have detected all potential adverse events related to CD if the patient had been discharged.
Characteristics of the patients not eligible for early-dischargePatients classified as eligible for PPM implantationThe proposed algorithm identified 16% of our patients as eligible for PPM implantation. This prevalence is much higher than what was reported by Malebranche et al. (6%).17 Compared to this study, our patients presented a higher Society of Thoracic Surgery mortality score, the type of valve used was more often a self-expandable valve and pre-existing LBBB and RBBB were more frequent. Also, the incidence of patients assigned to group 5 (17% vs 8%) and group 4 (36% vs 22%) was higher in our study, with less patients allocated to group 1 (35% vs 45%). These differences may explain the higher number of patients classified as suitable for PPM implantation in our cohort.
Almost all patients classified as eligible for PPM implantation (97%) actually underwent PPM implantation during hospital stay, a finding comparable to the study of Malebranche et al.,17 in which all patients classified as candidates for PPM implantation underwent PPM implantation. Thus, the algorithm seems to have the potential to correctly identify patients with a clear indication for PPM implantation.
Strategies and future perspectives for patients classified as at higher risk for HAVB/CHBOf the 18% of the patients classified as high risk for HAVB/CHB, 30% ended up being submitted to PPM implantation within 30 days after TAVI. PPM implantation occurred on average, 6 days after TAVI, and the 6-month atrial and ventricular pacing rate was 37% and 3%, respectively.
The optimal duration and mode of monitoring and the indication for PPM implantation in patients at higher risk for HAVB/CHB are not fully established. The 2019 consensus document proposes different strategies to manage CD in these patients: a) continuous ECG monitoring, b) electrophysiological (EP) study or c) PPM implantation; these recommendations are similar to those of the recent guidelines on cardiac pacing in patients undergoing TAVI.23
In our cohort, patients at higher risk for HAVB/CHB were mainly assigned to group 4 (58%) and group 1 (25%), with only 2.5% to group 5. The management of these heterogenous group of patients is controversial, making them candidates for longer surveillance, during hospital stay or in an outpatient setting, or until PPM implantation. Longer periods of inpatient monitoring increase arrhythmia detection, but also increase the length of hospital stay. An alternative approach to inpatient cardiac monitoring could be remote ambulatory monitoring. A recent prospective study24 revealed that ambulatory ECG monitoring 2 weeks before and after TAVI resulted in 7% planned PPM implantation, the great majority due to AVB. Reiter et al.25 also found that 12% of patients without persistent or recurrent CHB after TAVI developed CHB, assessed by implantable loop recorder, with onset between 2 days and 3 months after the procedure. In our cohort, the 30-day rate of PPM implantation for patients at higher risk for HAVB/CHB was only 30% and the 6-month pacing rate was low. In these patients, our threshold for PPM implantation is low; nevertheless, more than 2/3 of these patients ended up not needing PPM implantation. This suggests that a strategy of non-invasive continuous monitoring may be the most appropriate approach in this setting.
Another alternative approach for the management of CD in patients at higher risk for AHVB/CHB is EP study. Intraprocedural prolongation of HV interval during EP study was a significant predictor of delayed AVB after TAVI.26 Knecht et al.27 showed that 53% of the patients with new onset LBBB after TAVI that underwent EP study and presented a HV interval>55ms developed HAVB during 12 months follow-up. In our study, almost 60% of the patients at higher risk for HAVB/CHB were classified in group 4 (new onset LBBB after TAVI); however, none underwent routine EP study. The EP study is an invasive procedure with increased risks, particularly in older and fragile patients, it prolongs hospital stay, and currently, no standardized protocol is available to identify which patients with LBBB are at higher risk for HAVB, making this strategy less appealing in this population.
Overall, the 30-day rate of PPM implantation in our cohort was high (24%). As the use of TAVI expands to include younger and low-risk patients, it will become increasingly important to understand the impact of PPM implantation after TAVI. Conflicting data exists regarding the clinical impact of PPM implantation after TAVI. The SWEDEHEART28 observational study found no differences in long-term survival, heart failure or endocarditis between patients who did and did not undergo PPM implantation. In contrast with those findings, other studies showed an increase in all-cause mortality29 and decline in left ventricle ejection fraction30 in patients with PPM implantation after TAVI.
Nonetheless, the management of patients at higher risk for HAVB/CHB is still a matter of debate, and all efforts to minimize unnecessary PPM implantation are warranted. Although recent pacing guidelines23 proposed an approach for these patients similar to the consensus document, these are still based on class IIa and IIb indications. Interestingly, one study31 reported that selective prophylactic PPM implantation in patients with baseline RBBB (RBBB with a QRS≥150ms or bifascicular block or RBBB and first-degree AVB with PR≥220ms) reduced TAVI procedural time and hospital length of stay and prevented early cardiac rehospitalization due to CHB. However, there is currently no evidence to support PPM implantation as a “prophylactic” measure before TAVI in asymptomatic patients or in patients who do not meet the standard indications for PPM implantation.23
Whether a strategy of prolonged non-invasive electrocardiographic monitoring before or after TAVI, in or out of the hospital, or whether more disruptive strategies based on implantable devices for cardiac monitoring, systematic EP studies, or prophylactic PPM implantation remains to be determined, and further studies are warranted.
Study limitationsThis is a retrospective analysis, subject to the limitations of this type of study. Patients were managed at the physician's discretion and not as proposed by the algorithm which was implemented later and did not interfere with the medical decision. The indication for PPM implantation was at the discretion of the treating physicians, and therefore different criteria may have been used for decision about PPM implantation among patients subsequently classified as eligible or non-eligible for ED, introducing potential bias and limiting the validity of the findings. A prospective study design with a standardized protocol for PPM implantation would provide more robust evidence.
This study has a relatively small sample size, which could limit the generalizability of the findings, especially in multiple subgroups. Despite this, the main findings of our study were in agreement within a larger cohort from the study of Malebranch et al.17
Finally, electrophysiological study was not performed as well as systematic electrocardiographic monitoring after discharge.
ConclusionsIn this real-world cohort of patients with CD post-TAVI, the proposed algorithm was able to identify a significant number of low-risk patients, suitable for ED. Additionally, the ED strategy seemed to be safe. According to the algorithm, 97% of the patients eligible for PPM implantation effectively underwent PPM implantation. Thirty percent of patients at higher risk for HAVB/CHB underwent PPM implantation; nevertheless, the 6-months pacing rate was low.
Thus, our study reinforces that the proposed algorithm allows to ED a significant number of patients, seems to be safe and enables to identify patients with a clear indication for PPM. However, the algorithm still identifies a significant number of patients at high risk for HAVB/CHB whose management is not fully established and require further validation.
- •
The management of CD after TAVI, the criteria for PPM implantation and the timing for discharge are not clearly established.
- •
A recent interdisciplinary expert consensus document has been published, providing guidelines for the management of CD after TAVI.
- •
The study aimed to evaluate the performance of the 2019 consensus algorithm on the management of CD after TAVI, in a real-world cohort.
- •
The study supported the findings that the algorithm has enabled a significant number of patients with CD post-TAVI to be discharged early and safely and to identify patients with a clear indication for PPM implantation.
- •
However, in high-risk patients, the algorithm does not provide a guiding strategy.
The authors report that there has been no financial support for this work.
Authors’ contributionsAll authors listed on the title page meet authorship criteria, have read the manuscript, attest to the validity and legitimacy of the data and agree to its submission to REC: CardioClinics.
Conflicts of interestThe authors have no conflicts of interest to declare.
Abbreviations: CD: conduction disturbances; CHB: complete heart block; ED: early-discharge; HAVB: high-degree atrioventricular block; PPM: permanent pacemaker; TAVI: transcatheter aortic valve implantation.