To study the incidence, predictors and prognosis of follow-up de novo heart failure (HF) after discharge for acute myocardial infarction (AMI) in patients with left ventricular ejection fraction (LVEF) ≥50% and without previous history of HF.
MethodsRetrospective multicenter registry that includes 2158 consecutive patients discharged for AMI, from January 2011 to December 2015, with LVEF ≥50% and without history of HF. The association between clinical variables and the development of de novo HF was assessed by Fine–Gray proportional hazards regression analysis, accounting for death as a competing episode. The adjusted effect of post-discharge HF on subsequent mortality was investigated using extended Cox proportional hazards model.
ResultsDuring follow-up (20.1±11.8 months), 60 patients (2.8%) had HF admissions, with a cumulative incidence function of 1.7 per 100 patient-year. Age ≥70 years, hypertension, atrial fibrillation, and renal dysfunction were independent risk factors for follow-up HF. The HF incidence rate after surviving AMI increased with a higher number of risk factors, from 0.1% in patients with 0, to 1.8%, 3.8%, 15.3%, and 19% in patients with 1, 2, 3, and 4 risk factors, respectively (P <.0001). Patients who experience follow-up new-onset HF had a 3.77-fold increased risk of death compared with other AMI survivors.
ConclusionsDe novo HF after hospital discharge for ACS in patients with LVEF ≥50% is an independent predictor of follow-up death. Fortunately, it is an infrequent post-AMI complication and is easily predictable the combination of 4 clinical risk factors.
Estudiar la incidencia, los predictores y el pronóstico de la insuficiencia cardiaca (IC) de novo tras el alta de un infarto agudo de miocardio (IAM) en pacientes con fracción de eyección del ventrículo izquierdo (FEVI) ≥50%, sin historia previa de IC.
MétodosRegistro multicéntrico retrospectivo que incluyó a 2.158 pacientes dados de alta por IAM entre enero de 2011 y diciembre de 2015 con FEVI≥50%, y sin antecedentes de IC. Para la asociación entre las variables clínicas y el desarrollo de la IC de novo se empleó un modelo de riesgos competitivos de Fine-Gray, siendo la muerte el evento competitivo.
ResultadosDurante el seguimiento (20,1±11,8 meses), 60 pacientes (2,8%) ingresaron por IC, con una incidencia acumulada de 1,7 por 100 pacientes/año. Los factores de riesgo independientes en el seguimiento fueron: edad ≥70 años, hipertensión, fibrilación auricular y enfermedad renal. La tasa de incidencia de IC tras sobrevivir a un IAM se incrementa con el número de factores de riesgo: desde el 0,1% para 0 hasta el 1,8, 3,8, 15,3 y 19% en pacientes con 1, 2, 3 y 4 factores, respectivamente (p<0,0001). Además, los pacientes con IC de novo tienen 3,77 veces más riesgo de muerte, comparado con el resto.
ConclusionesLa IC de novo tras el alta de IAM en pacientes con FEVI ≥50% es un predictor independiente de muerte en el seguimiento. Afortunadamente, es una complicación infrecuente post-IAM y es fácilmente predecible con la combinación de 4 factores de riesgo clínicos.
Heart failure (HF) is common after acute myocardial infarction (AMI), which is considered to be one of its major precursors,1 and has been associated with excess mortality.2,3 The risk of experiencing post-infarction HF varies considerably across the spectrum of AMI survivors. Most of HF episodes happen in patients with left ventricular systolic dysfunction and during the hospitalization by AMI.4,5 In fact, reduced left ventricular ejection fraction (LVEF) is the single most common cause of HF after AMI.6 However, no studies have specifically analyzed the incidence of post-infarction HF in patients with preserved LVEF. A better understanding of the factors involved in the eventual development of HF in AMI survivors would help to generate potential mechanistic information. It also would enable a better identification of high-risk patients who may benefit from implementation of more intensive preventive measures.
Improvements in the treatment of AMI, especially with the coronary revascularization, have led to greater myocardial salvage and a reduced extent of ventricular injury.7 Because of that, most of current AMI patients have preserved LVEF. In this subgroup of patients, there are no studies about the incidence and predictors of follow-up HF after discharge for AMI. The objective of this analysis was to provide scientific evidence in this issue, attempting to do a quantitative evaluation of the incidence and factors that predict the development of de novo HF in post-AMI patients with preserved LVEF, together to its prognostic impact in terms of mortality.
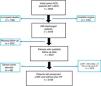
MethodsPatient populationThe data analyzed in this study were obtained from a merged retrospective clinical registry including all patients consecutively admitted to the cardiology department of 2 tertiary hospitals from 2011, January to 2015, December, with the primary and definitive diagnosis of acute coronary syndrome, underwent coronary angiography (n=4653). From them, we selected those with AMI (n=3908). Patients who died in hospital (n=199) and those with no data on vital status and/or on HF status during follow-up (n=202) were excluded. Since our objective was to study the incidence and predictor of de novo HF in patients with preserved LVEF, we excluded patients who presented prior history of HF, Killip ≥II at admission, in-hospital HF during AMI admission, and LVEF <50%. Thus, the final cohort of the present study consisted of 2158 patients. The flow chart can be seen in Fig. 1.
The present study complied with the Declaration of Helsinki and was approved by the hospital ethic committee (Hospital Álvaro Cunqueiro). Data on demographic and clinical characteristics, complementary test results, angiographic parameters, in-hospital events as well as treatment at discharge were collected in detail by trained cardiologists.
Study endpoint, definitions, and follow-upThe ascertainment of HF status and death during follow-up was carried out, between September and October of 2016. We reviewed the electronic medical record and all the medical attendances and hospital records. Definition of AMI was based on the European Society of Cardiology third universal definition of myocardial infarction. HF diagnosis was based on the presence of both symptoms and signs due to a documented structural or functional cardiac abnormality. Follow-up de novo HF was defined as any hospital admission by HF after hospital discharge.
Statistical analysisCategorical variables were presented as frequency values and continuous variables were presented as mean±standard deviation. Differences in baseline characteristics were compared using the Student t-test for continuous variables and the chi-square test for categorical variables. The rate of de novo post-discharge HF, adjusted for death as a competitive risk event, was calculated using the Fine–Gray method and reported as cumulative incidence function.
To assess the association between clinical variables and the development of de novo HF, we performed a Fine–Gray proportional hazards regression analysis, accounting for death as a competing episode as was previously mentioned. The variables associated with post-discharge HF (P<.05) were included in a multivariate model. Those variables were: age, female sex, hypertension, diabetes mellitus, ST-elevation myocardial infarction, hemoglobin at admission, Chronic Kidney Disease Epidemiology Collaboration at admission <60mL/min/1.73m2, atrial fibrillation (AF), multivessel coronary artery disease, complete revascularization and statin therapy. The adjusted hazard of HF was expressed as subhazard ratios (sHR) with their corresponding 95% confidence intervals (95%CI).
As HF status change over time, the crude and adjusted effect of post-discharge HF on subsequent all-cause mortality were investigated using extended Cox proportional hazards models, a flexible modeling approach that relaxes the proportionality assumption and allows for covariate effects to vary over time. Cox models were adjusted using the following covariates that were either statistically significant or plausibly related to mortality.
All P <.05 were accepted as statistically significant. Statistical analysis was performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, United States) and STATA version 13.0 (Stata Corp LP., TX, United States).
ResultsBaseline characteristics and follow-up eventsThe basal characteristics of the population studied are presented in Table 1. From the 2158 AMI patients with preserved LVEF, 60 (2.8%) died during follow-up (20.1±11.8 months), and 59 (2.8%) were diagnosed of de novo HF (cumulative incidence function: 1.7 per 100 patient-year, 95%CI, 1.3–2.1). Median time to HF diagnosis was 5.6 months (interquartile range: 2.0–13.6 months).
Baseline characteristics.
| Variables | Total population |
|---|---|
| Age, years | 63.3±12.8 |
| Female sex, % | 514 (23.8%) |
| Diabetes mellitus, % | 567 (26.3%) |
| Hypertension, % | 1275 (59.1%) |
| Peripheral artery disease, % | 120 (5.6%) |
| ST-elevation myocardial infarction, % | 902 (41.8%) |
| Hemoglobin at admission, g/dL | 14.3±1.7 |
| CKD-EPI at admission <60mL/min/1.73m2 | 142 (6.6%) |
| Atrial fibrillation, % | 168 (7.8%) |
| Mitral regurgitation grade 3–4, % | 39 (1.8%) |
| Multivessel coronary artery disease, % | 913 (42.3%) |
| PCI, % | 1657 (76.8%) |
| CABG, % | 82 (3.8%) |
| Complete revascularization, % | 1282 (59.4%) |
| AAS, % | 2100 (97.3%) |
| Clopidogrel, % | 1280 (59.3%) |
| Ticagrelor, % | 430 (19.9%) |
| Prasugrel, % | 250 (11.6%) |
| OAC, % | 160 (7.4%) |
| Beta-blocker, % | 1178 (82.4%) |
| ACEi/ARB, % | 71.5 |
| Statin, % | 95.7 |
AAS, antiaggregant; ACEi/ARB, angiotensin-converting enzyme inhibitor/aldosterone receptor blockade; CABG, coronary artery bypass surgery; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; OAC, oral anticoagulant; PCI, percutaneous coronary intervention.
Patients with post-myocardial infarction HF were older, more likely to be women, to have AF, anemia, and worse cardiovascular risk profile. They also presented at hospital less frequently as ST-segment elevation myocardial infarction (STEMI), but had more commonly multivessel disease although underwent incomplete revascularization procedures (Table 2).
Uni and multivariate Fine–Gray analysis for post-discharge heart-failure.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| sHR | 95%CI | P | sHR | 95%CI | P | |
| Age, per 1 year | 1.08 | 1.05–1.10 | <.001 | 1.04 | 1.01–1.07 | .007 |
| Female sex, % | 2.83 | 1.69–4.72 | <.001 | 1.18 | 0.59–2.37 | .633 |
| Diabetes mellitus, % | 2.40 | 1.44–4.00 | .001 | 1.38 | 0.75–2.54 | .293 |
| Hypertension, % | 4.32 | 2.05–9.11 | <.001 | 3.09 | 1.05–9.06 | .040 |
| ST-elevation myocardial infarction, % | 0.42 | 0.23–0.77 | .005 | 0.96 | 0.50–1.85 | .909 |
| Hemoglobin at admission, g/dL | 0.69 | 0.61–0.78 | <.001 | 0.89 | 0.72–1.10 | .270 |
| CKD-EPI at admission <60mL/min/1.73m2 | 5.12 | 2.86–9.20 | <.001 | 2.30 | 1.05–5.04 | .037 |
| Atrial fibrillation, % | 8.87 | 5.28–14.90 | <.001 | 3.34 | 1.67–6.66 | .001 |
| Multivessel coronary artery disease, % | 2.06 | 1.03–2.88 | .040 | 1.33 | 0.73–2.42 | .356 |
| Complete revascularization, % | 0.51 | 0.29–0.91 | .021 | 1.01 | 0.56–1.80 | .984 |
| Statin, % | 0.37 | 0.16–0.85 | .019 | 0.39 | 0.13–1.19 | .098 |
CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; sHR, hazard ratios.
Treatment at discharge with beta-blockers (83.1% vs 82.4%, P=.893) and angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (70.7% vs 71.6%, P=.882) was similar between groups according to the HF status during follow-up, however patients who developed HF were less often treated with statins (89.8% vs 96.0%, P=.019).
Moderate–severe mitral regurgitation (grade ≥III) after AMI was not associated with higher incidence of post-discharge HF (1.9% vs 1.6%; P=.902).
Table 2 shows the multivariate analysis. Age (sHR, 1.02; 95%CI, 1.01–1.07; P=.007), hypertension (sHR, 3.09; 95%CI, 1.05–9.06; P=.040), renal dysfunction as glomerular filtrate rate estimated by Chronic Kidney Disease Epidemiology Collaboration equation <60mL/min/1.73m2 (sHR, 2.30; 95%CI, 1.05–5.04; P=.037), and AF (sHR, 3.34; 95%CI, 1.67–6.66; P=.001), were independent predictors of HF development after discharge. The c-statistic of the model was 0.74±0.04. An age of ≥70 years was the most appropriate cutoff for predicting post-AMI HF, with sensitivity 67.8% and specificity 68.6%.
Four out of the 59 patients who developed post-discharge HF, also suffered reinfarction during follow-up. After excluding these 4 patients, there were not significant changes in the multivariate model showed in Table 1.
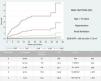
In order to facilitate the post-discharge HF risk estimation, we build a simple system considering the presence of one or more of the 4 independent clinical conditions retained in the multivariate model. A total of 726 patients (33.6%) had 0 risk factors, 814 (37.7%) had 1, 453 (21.0%) had 2, 144 (6.7%) had 3, and only 21 (1.0%) had all 4 risk factors. The HF incidence rate increase from 0.1% in patients without any them, to 1.8%, 3.8%, 15.3%, and 19% in patients with 1, 2, 3, and 4 risk factors, respectively (P <.0001 for trend across strata; Fig. 2).
Follow-up HF and mortalityDe novo HF after hospital discharge by AMI was related to higher risk of subsequent mortality (Fig. 3; HR 6.82; 95%CI, 3.21–14.47; P<.001). After adjusting for the other variables associated with mortality, post-AMI HF remains as significant predictor of follow-up mortality (HR 3.77; 95%CI, 1.65–8.65; P=.002).
DiscussionSummary of findingsThe present study analyzes the incidence, predictors and prognosis of HF development after hospital discharge by AMI in Killip I patients with preserved LVEF and without prior HF or HF during hospitalization. Using data of a retrospective contemporary registry from 2 tertiary Spanish hospitals, we have reported a cumulative incidence function of post-discharge HF of 1.7%. Most of HF episodes happened in the first 6 months after AMI. Although the HF rate was low, we have found a simple predictive model based on the combination of 4 clinical HF risk factors: age ≥70 years, hypertension, AF and renal dysfunction. With this model, we can accurately stratify the risk of developing post-AMI HF in this particular subgroup of patients (LVEF ≥50% and without history of HF). Moreover, follow-up HF after discharge for AMI was an independent predictor of mortality, with an increase of more than 3 times the risk of death.
Interpretation of study findingsWith the widespread use of percutaneous coronary intervention, the percentage of patients with post-infarction ventricular systolic dysfunction has decreased.8 The most important risk factor for predicting HF in follow-up after an AMI is the presence of ventricular systolic dysfunction. Patients with preserved LVEF after an AMI are considered as low risk for de novo HF.6 However, until our study, and based on our knowledge, there were no specific studies available that analyzed the true incidence and predictors of de novo HF in this subgroup. This study analyzes this issue for the first time, reporting very low rates of post-AMI de novo HF (1.7 per 100 patient-year). In the era prior to percutaneous coronary intervention, the annual HF rate ranged from 20% to 30% during the first year after AMI.9 In the current era of coronary revascularization, the rate of post-AMI HF decreased significantly.10,11 In general, overall HF rates in the first year after AMI range from 5 to 15%,12,13 with most of these HF events happened during AMI hospitalization. In certain risk groups, such as patients with complicated AMI with ventricular dysfunction or HF, annual HF rates may be as high as 15%, as reported in the VALIANT (Valsartan in Acute Myocardial Infarction Trial) study.14 In patients without previous history of HF and with preserved LVEF (≥50%), rates are markedly lower, although no study specifically analyzed these patients.
Our study analyzes incidence of HF after hospital discharge by AMI, in a contemporary cohort of patients with LVEF ≥50% and without HF, all of whom underwent catheterization, with 77% of percutaneous coronary intervention, 82% of beta-blocker prescription, and 71% of Angiotensin-Converting Enzyme Inhibitors/Angiotensin II Receptor Blockers prescription. We reported a rate of HF of 2.8% in a mean time of 20 months, concentrating half of the events in the first 6 months. The high rate of revascularization as well as the high rate of optimal medical therapy could explain the low incidence of de novo post-AMI HF, that is consistent with the HF rate reported in the CARE trial.6
The most interesting issue is that the vast majority of these events can be predicted. In our study, 4 variables (age ≥70 years, hypertension, AF, and renal dysfunction) were identified as independent predictors of post-discharge HF. With the combination of these factors, we can stratify the risk of post-AMI HF, with a rate of 0.1% during the mean follow-up in patients without risk factors and close to 20% in high-risk patients (this is, patients with the 4 risk factors). Previous studies have demonstrated several important predictors of HF following AMI, including older age, comorbid illnesses, and factors related to the extent of myocardial injury, such as Killip classification and left ventricular dysfunction.6 But these studies analyze all spectrums of AMI patients, as in the case of LONGEVO-SCA registry in which fragility predicts mortality in elderly patients.15 Although there are no studies that have specifically analyzed the predictors of de novo HF after AMI in patients with preserved LVEF, the risk factors are common to other prior studies that studied follow-up HF in total population after AMI, like age, hypertension, AF and renal dysfunction.
Our study showed that the development of HF after AMI increases steeply with age. There was a 5% increase in HF risk with each year, similar to the study by Hellermann et al., which showed an increase of 4%.16 The relation of age with HF and cardiac fibrosis is something already known and previously reported. In our study the cut-off point with a better predictive capacity was 70 years. The study of Torabi et al.17 found a progressive increase in the risk of post-AMI HF with the age, establishing cut-off points at 65 and 75 years. And Ezekowitz et al.2 reported a very high risk of HF development in patients ≥65 years of age hospitalized for a first myocardial infarction without HF history (75% in the 5 years).
Other risk factor for de novo HF was hypertension. We found a 3-times fold increase in the risk of post-AMI HF in hypertensive patients. This association was also previously reported in previous studies, with hypertension as an independent predictor of post-AMI HF.6 One possible pathophysiological link for this association is based on the interaction between hypertension with age, neurohumoral activation and early ventricular remodeling to confer greater risk of HF after myocardial infarction.18
Renal dysfunction was also a common risk factor for HF development after AMI. In our study, there is a 5-times fold increased risk in patients with Chronic Kidney Disease Epidemiology Collaboration <60mL/min/1.73m2. It is known that patients with impairment in renal function are at increased risk for adverse cardiovascular outcomes after AMI, included HF. In this way, renal disease is associated with a variety of cardiac alterations including left ventricular hypertrophy and/or dilation, and reduction in diastolic function.19 These cardiac changes are partly explained by factors associated with renal decline, including anemia, oxidative stress, derangements in calcium–phosphate homeostasis, inflammation, and conditions promoting coagulation, all of which are associated with accelerated atherosclerosis and endothelial dysfunction. Other non-conventional risks that progressively increase with renal decline include albuminuria, proteinuria, homocysteinemia, and elevated uric acid levels.20
Atrial fibrillation was regarding to be the strongest predictor of de novo HF in post-AMI patients with preserved LVEF. Other studies have previously reported the association of AF with follow-up HF in the total population of post-AMI patients.21 There are many explanations for this physiopathological link: AF may facilitate the development or progression of HF in several ways.22 The increase in resting heart rate and the exaggerated heart rate response to exercise, result in shorter diastolic filling time, leading to a reduction in cardiac output. This is further affected by the irregularity of the ventricular response: The reduction in left ventricular filling during short cycles is not completely compensated for by increased filling during longer cycles. The loss of effective atrial contractile function also contributes, even more importantly in patients with diastolic dysfunction.23 Restoration of sinus rhythm improves cardiac output, exercise capacity, and maximal oxygen consumption.
Regarding to the prognostic effect of follow-up HF after hospital discharge for AMI, our results were consistent with other studies conducted in general population and in those with reduced LVEF.1,24,25 It was previously proposed that HF after myocardial infarction not only increases mortality, but also the associated risk of other prognostic factors, such as cancer, diabetes mellitus, and chronic renal failure.2,26 Regardless of the mechanisms involved in this association, an increasing body of evidence supports the concept of HF as a sentinel condition which might reflect end-stage chronic diseases.1 The new conclusion of our study is that this forecast effect is also seen in patients with preserved LVEF after AMI.
Clinical implicationsOur study provides a representative and contemporary picture of the post-discharge HF risk after AMI in daily practice patients with preserved LVEF and no history of HF.
From the clinical viewpoint, quantification of HF admission risk at the time of hospital discharge provides an opportunity to enroll high-risk patients into proactive care management programs, reducing hospitalization costs and improving the quality of care as well as the patient's functional status. Our simple model, based in 4 clinical variables, gives information early enough, prior to discharge, to trigger a transitional care intervention with well-scheduled visits. More intensive follow-up and patient care, in high risk HF patients, aim to reduce post-discharge HF death rate.
Limitations and strengthsSome limitations should be acknowledged in interpreting these data. These results emanate from a retrospective study including patients with AMI undergoing coronary angiography during the index hospitalization in 2 Spanish tertiary hospitals, with the limitations inherent to this type of design. Patients with conservative management (this is, without coronary angiography) were not included, although those patients have a special relevance in this issue. In addition, although a multivariable model to adjust for potential confounders was used, unmeasured or residual confounding factors may persist. In this sense, information about changes in LVEF after hospital discharge were not available neither emergency visits due to mild HF which did not require hospitalization.
Moreover, we have no data regarding medication compliance and socioeconomic and educational variables, which can affect the occurrence of HF in follow-up. And we also do not have data about biomarkers with utility in HF, such as B-type natriuretic peptide, mid-regional pro-atrial natriuretic peptide, mid-regional pro-adrenomedullin, soluble ST2, or Galectin-3.
ConclusionsDe novo HF after AMI in contemporary patients with preserved LVEF is infrequent, but easily predictable. Age ≥70 years, hypertension, AF, and renal dysfunction, are the independent risk factors for developing post-AMI HF in this population. Development of HF after hospital discharge is a strong risk factor for all-cause mortality.
Postinfarction HF is an entity related to high mortality; it is usually more prevalent among those patients with previous ventricular dysfunction. However, no studies have specifically analyzed its incidence in patients with preserved LVEF.
Currently, the management of acute AMI has improved. So, the number of patients with preserved LVEF is increasing. Identifying the high-risk group for de novo HF can help to intensify preventive measures in patients with AMI.
Does it contribute anything new?Postinfarction de novo HF, in patients with previous preserved EF, is a rare complication (cumulative incidence function of 1.7 per 100 patients-year). However, it is easy predictable with 4 risk factors prior to hospital discharge: age ≥70 years, arterial hypertension, atrial fibrillation and renal dysfunction. Postinfarction de novo HF is an independent predictor of mortality (HR 3.77; 95%CI, 1.65–8.65; P=.002).
None.
Abbreviations: AF, atrial fibrillation; AMI, acute myocardial infarction; HF, heart failure; LVEF, left ventricular ejection fraction.