In recent years, antigen carbohydrate 125 (CA125) has emerged as a promising biomarker for monitoring congestion, guiding diuretic therapy, and risk stratification of patients with heart failure (HF).1 Recent evidence of novel applications of CA125 has come to light, such as identifying the patient's congestion phenotype to tailor diuretic therapy.2,3 Nonetheless, several factors should be considered to interpret CA125 correctly. In this article, we report a case series of 6 patients that illustrate the applicability of CA125 in different clinical scenarios. Table 1 summarizes their clinical characteristics.
Clinical characteristics of the six case studies.
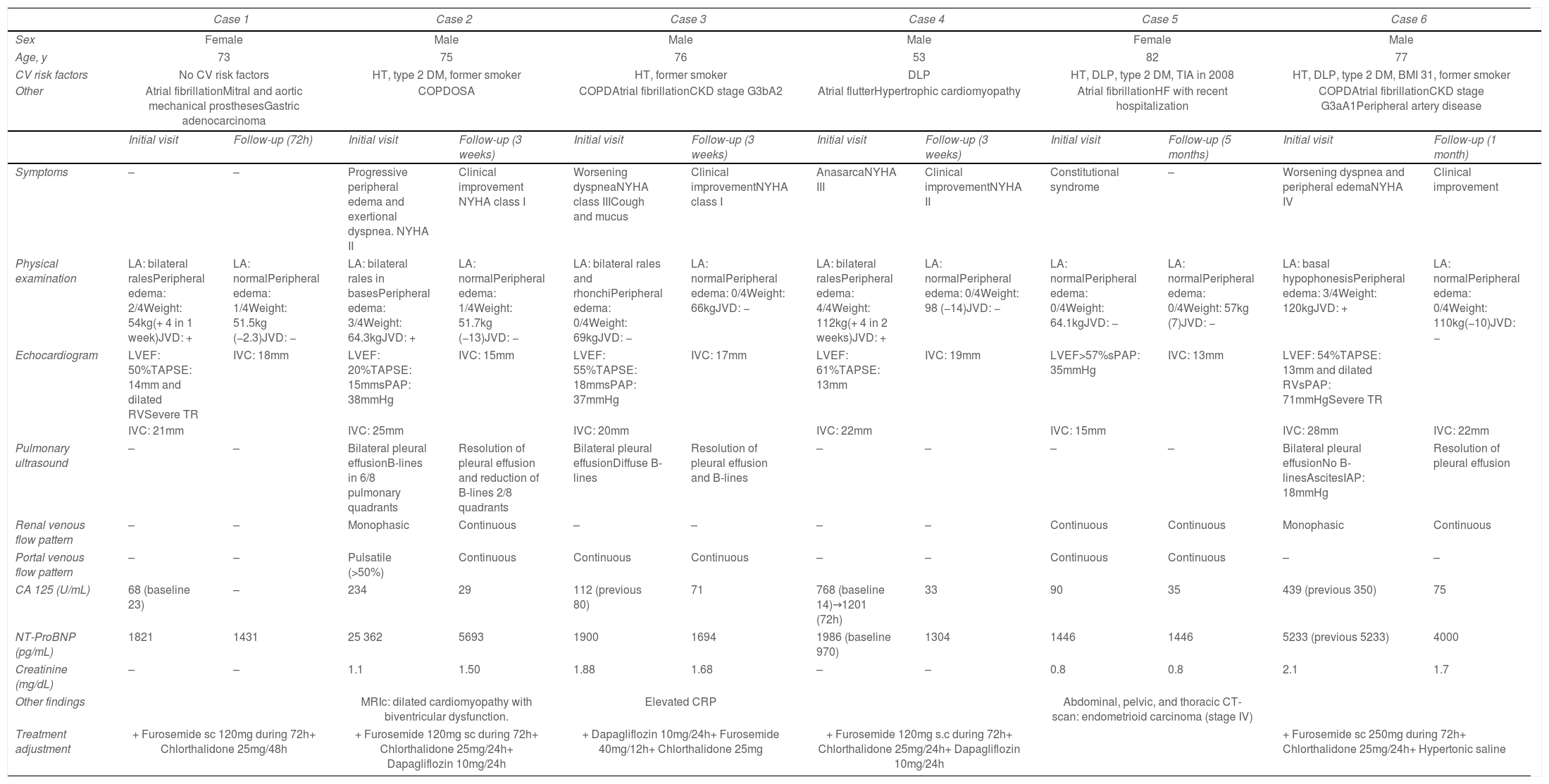
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Female | Male | Male | Male | Female | Male | ||||||
| Age, y | 73 | 75 | 76 | 53 | 82 | 77 | ||||||
| CV risk factors | No CV risk factors | HT, type 2 DM, former smoker | HT, former smoker | DLP | HT, DLP, type 2 DM, TIA in 2008 | HT, DLP, type 2 DM, BMI 31, former smoker | ||||||
| Other | Atrial fibrillationMitral and aortic mechanical prosthesesGastric adenocarcinoma | COPDOSA | COPDAtrial fibrillationCKD stage G3bA2 | Atrial flutterHypertrophic cardiomyopathy | Atrial fibrillationHF with recent hospitalization | COPDAtrial fibrillationCKD stage G3aA1Peripheral artery disease | ||||||
| Initial visit | Follow-up (72h) | Initial visit | Follow-up (3 weeks) | Initial visit | Follow-up (3 weeks) | Initial visit | Follow-up (3 weeks) | Initial visit | Follow-up (5 months) | Initial visit | Follow-up (1 month) | |
| Symptoms | – | – | Progressive peripheral edema and exertional dyspnea. NYHA II | Clinical improvement NYHA class I | Worsening dyspneaNYHA class IIICough and mucus | Clinical improvementNYHA class I | AnasarcaNYHA III | Clinical improvementNYHA II | Constitutional syndrome | – | Worsening dyspnea and peripheral edemaNYHA IV | Clinical improvement |
| Physical examination | LA: bilateral ralesPeripheral edema: 2/4Weight: 54kg(+ 4 in 1 week)JVD: + | LA: normalPeripheral edema: 1/4Weight: 51.5kg (−2.3)JVD: − | LA: bilateral rales in basesPeripheral edema: 3/4Weight: 64.3kgJVD: + | LA: normalPeripheral edema: 1/4Weight: 51.7kg (−13)JVD: − | LA: bilateral rales and rhonchiPeripheral edema: 0/4Weight: 69kgJVD: − | LA: normalPeripheral edema: 0/4Weight: 66kgJVD: − | LA: bilateral ralesPeripheral edema: 4/4Weight: 112kg(+ 4 in 2 weeks)JVD: + | LA: normalPeripheral edema: 0/4Weight: 98 (−14)JVD: − | LA: normalPeripheral edema: 0/4Weight: 64.1kgJVD: − | LA: normalPeripheral edema: 0/4Weight: 57kg (7)JVD: − | LA: basal hypophonesisPeripheral edema: 3/4Weight: 120kgJVD: + | LA: normalPeripheral edema: 0/4Weight: 110kg(−10)JVD: − |
| Echocardiogram | LVEF: 50%TAPSE: 14mm and dilated RVSevere TR | IVC: 18mm | LVEF: 20%TAPSE: 15mmsPAP: 38mmHg | IVC: 15mm | LVEF: 55%TAPSE: 18mmsPAP: 37mmHg | IVC: 17mm | LVEF: 61%TAPSE: 13mm | IVC: 19mm | LVEF>57%sPAP: 35mmHg | IVC: 13mm | LVEF: 54%TAPSE: 13mm and dilated RVsPAP: 71mmHgSevere TR | |
| IVC: 21mm | IVC: 25mm | IVC: 20mm | IVC: 22mm | IVC: 15mm | IVC: 28mm | IVC: 22mm | ||||||
| Pulmonary ultrasound | – | – | Bilateral pleural effusionB-lines in 6/8 pulmonary quadrants | Resolution of pleural effusion and reduction of B-lines 2/8 quadrants | Bilateral pleural effusionDiffuse B-lines | Resolution of pleural effusion and B-lines | – | – | – | – | Bilateral pleural effusionNo B-linesAscitesIAP: 18mmHg | Resolution of pleural effusion |
| Renal venous flow pattern | – | – | Monophasic | Continuous | – | – | – | – | Continuous | Continuous | Monophasic | Continuous |
| Portal venous flow pattern | – | – | Pulsatile (>50%) | Continuous | Continuous | Continuous | – | – | Continuous | Continuous | – | – |
| CA 125 (U/mL) | 68 (baseline 23) | – | 234 | 29 | 112 (previous 80) | 71 | 768 (baseline 14)→1201 (72h) | 33 | 90 | 35 | 439 (previous 350) | 75 |
| NT-ProBNP (pg/mL) | 1821 | 1431 | 25 362 | 5693 | 1900 | 1694 | 1986 (baseline 970) | 1304 | 1446 | 1446 | 5233 (previous 5233) | 4000 |
| Creatinine (mg/dL) | – | – | 1.1 | 1.50 | 1.88 | 1.68 | – | – | 0.8 | 0.8 | 2.1 | 1.7 |
| Other findings | MRIc: dilated cardiomyopathy with biventricular dysfunction. | Elevated CRP | Abdominal, pelvic, and thoracic CT-scan: endometrioid carcinoma (stage IV) | |||||||||
| Treatment adjustment | + Furosemide sc 120mg during 72h+ Chlorthalidone 25mg/48h | + Furosemide 120mg sc during 72h+ Chlorthalidone 25mg/24h+ Dapagliflozin 10mg/24h | + Dapagliflozin 10mg/24h+ Furosemide 40mg/12h+ Chlorthalidone 25mg | + Furosemide 120mg s.c during 72h+ Chlorthalidone 25mg/24h+ Dapagliflozin 10mg/24h | + Furosemide sc 250mg during 72h+ Chlorthalidone 25mg/24h+ Hypertonic saline | |||||||
BMI: body mass index; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; CT: computed tomography; CV: cardiovascular; DLP: dyslipidemia; DM: diabetes mellitus; ECG: electrocardiogram; HF: heart failure; HT: hypertension; IVC: inferior vena cava; JVD: jugular vein distention; LA: lung auscultation; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MRIc: cardiac magnetic resonance imaging; NT-proBNP: N-terminal brain natriuretic peptide; NYHA: New York Heart Association; OSA: obstructive sleep apnea; RV: right ventricle; sc, subcutaneous; sPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion; TIA: transitory ischemic attack; TR: tricuspid regurgitation.
CA125 is a large transmembrane glycoprotein synthesized by mesothelial cells that is widely used to monitor ovarian cancer. However, elevated values can also be found in other hydropic conditions such as HF.1 Several studies have confirmed its prognostic value mainly in acute HF (AHF) with reduced or preserved left ventricular ejection fraction (LVEF).1 In a sub-analysis of the BIOSTAT-CHF study,4 CA125 was strongly correlated with 1-year mortality risk and the composite of death and HF readmissions in 2516 patients with HF. Furthermore, it provided additive prognostic value over traditional risk factors such as N-terminal prohormone of brain natriuretic peptide (NT-ProBNP).
Patient 1 illustrates how to utilize the predictive power of CA125. She was a 73-year-old woman with chronic HF of valvular etiology in whom elevated CA125 levels (68U/mL; previous: 23U/mL) were detected in a routine follow-up, without meaningful variations in NT-ProBNP values (1821pg/mL previous: 1518pg/mL). The patient denied symptoms and had minimal peripheral edema. Echocardiography revealed a dilated inferior vena cava, dilated right ventricle (RV) with systolic dysfunction, and severe tricuspid regurgitation (TR). Based on these findings, despite being asymptomatic, we intensified diuretic therapy. On the follow-up visit, the patient had lost 2.3kg, and peripheral edema had resolved. Monitoring the trajectory of CA125 in this patient allowed for early detection of HF decompensation and timely treatment that helped prevent hospitalization.
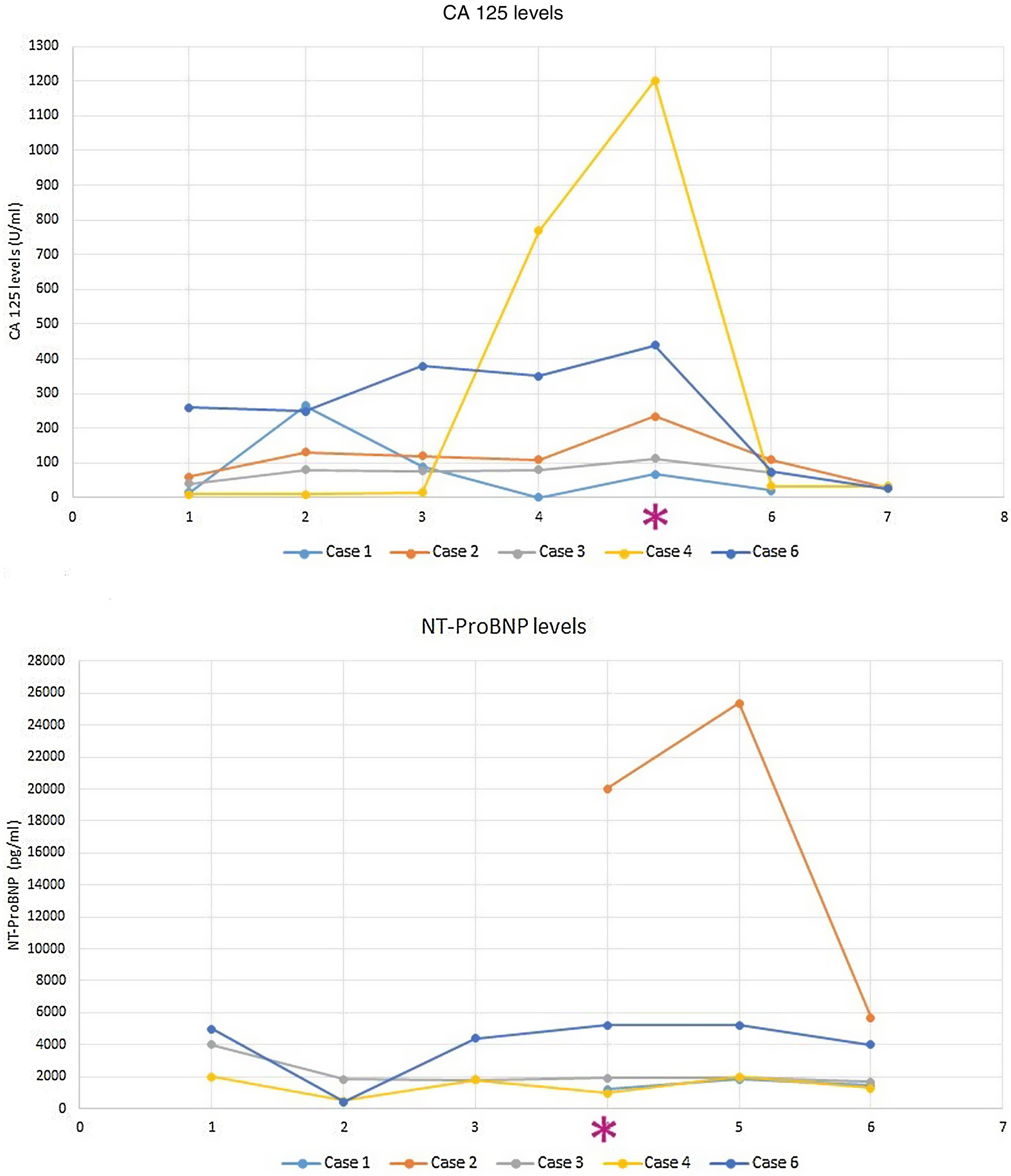
Conversely, CA125 has the potential for both monitoring and guiding HF treatment. The CHANCE-HF trial2 compared a CA125-guided therapy vs standard of care in 380 patients discharged for AHF. Diuretics were intensified when CA125 increased or persisted elevated and downtitrated when CA125 decreased. The study demonstrated that CA125-guided therapy was superior to standard of care in reducing the risk of 1-year death or AHF readmission. Fig. 1 illustrates how our patients’ CA125 levels decreased after the intensification of diuretic therapy in parallel with the improvement of signs/symptoms of congestion.
A good example of the use of CA125 to monitor diuretic therapy is patient 2, a 75-year-old man with a history of chronic obstructive pulmonary disease (COPD) who was admitted to hospital with AHF. Both NT-ProBNP (25362pg/mL) and CA125 were elevated (234U/mL). B-lines and bilateral pleural effusion were present on lung ultrasound. Intrarenal venous flow pattern (VFP) was monophasic, and portal VFP was pulsatile (>50%). During admission, the patient received high doses of intravenous furosemide (200mg/day), 15mg/day of tolvaptan, and 25mg/day of chlortalidone. Fourteen days after discharge, the patient had lost 13kg, congestion had disappeared, and ultrasound parameters of venous congestion (intrarenal and portal VFP) normalized. In accordance with the resolution of the signs and symptoms of congestion there was a substantial decrease in congestion biomarkers (CA125, 29U/mL; NT-ProBNP, 5693pg/mL), reaffirming our decision to down-titrate diuretic therapy.
A novel application of CA125 is to classify HF patients based on their congestion phenotype in intravascular or tissue congestion.5 This distinction is important because they have different pathophysiology and thus, require different treatment. CA125 helps identify HF patients with predominant tissue congestion that may benefit from aggressive diuretic therapy targeting interstitial fluid such as tolvaptan, sodium-glucose cotransporter-2 inhibitors (SGLT2i), or hypertonic saline. On the other hand, NT-ProBNP, jugular vein distention (JVD), and venous excess ultrasound score (VExUS) may help identify patients with predominant intravascular congestion who may not have an increase in total blood volume, but rather a dysregulation of blood distribution and, therefore, benefit from vasodilators and a conservative diuretic strategy.
Patient 3 exemplifies the tissue congestion phenotype. He was a 76-year-old man with right-side HF as a consequence of pulmonary hypertension and severe COPD who came to our clinic with progressive dyspnea due to a COPD exacerbation. The patient had no signs of intravascular congestion (NT-ProBNP was stable, no JVD and the VExUS was 0). However, CA125 was elevated (112U/mL; previous: 80U/mL) and he presented bilateral pleural effusion and B-lines on pulmonary ultrasound (signs of pulmonary tissue congestion). Identifying the patient's phenotype helped tailor the diuretic therapy he received, by intensifying oral diuretic therapy (furosemide 120mg/day plus chlorthalidone 25/48h) and adding dapagliflozin 10mg/day. Three weeks later, CA125 levels dropped to 71U/mL, and pleural effusion and B-lines disappeared.
When interpreting CA125 levels, it is important to consider 2 aspects. Firstly, CA125 has a long half-life (5.1–12 days)1 and may remain elevated or even increase the first days of decompensation. This is the case of patient 4, a 53-year-old man with hypertrophic cardiomyopathy and RV dysfunction who was referred to our outpatient clinic due to worsening symptoms and clinical evidence of volume overload. Blood analysis showed elevated CA125 (768U/mL). As the patient did not require hospital admission, ambulatory diuretic therapy intensification with 120mg/day of subcutaneous furosemide+25mg/day of chlorthalidone was initiated. Three days later, the patients had lost 12kg, and peripheral edema had resolved. Surprisingly, CA125 levels continued to increase (1201U/mL). Knowing the kinetics of CA125 and given the favorable course of other markers of decongestion, we decided not to modify treatment. The patient evolved favorably and CA125 concentrations eventually dropped. Therefore, serial measurements during the first days of admission are not recommended. A reasonable approach is to measure CA125 on admission and 7–10 days after the initial measurement. Secondly, CA125 should be evaluated with the patient's clinical context and other parameters of congestion. A good example is patient 5, an 82-year-old woman with a recent episode of AHF who was evaluated in our clinic 5 months after discharge. She had unintentionally lost 7kg, and CA125 had risen (35–90U/mL) despite absent signs of congestion (VExUS 0, absence of serosal effusion and NT-ProBNP similar to baseline). In light of this incongruency, we requested a computerized tomography scan and discovered a stage IV endometrioid carcinoma. A valuable lesson was learnt: increased CA125 levels in the absence of objective evidence of congestion (ideally with ultrasound techniques) must alert physicians to the possibility of malignancies.
Lastly, CA125 might outperform NT-ProBNP in patients with predominant RV dysfunction or systemic congestion, especially if they present severe TR.3,6 Moreover, CA125 levels are not influenced by age, LVEF, or eGFR (1), making it attractive for monitoring patients with concomitant heart and kidney dysfunction. By contrast, NT-ProBNP reflects predominant left-sided HF. Patient 6 represents a clinical scenario in which CA125 has demonstrated to be superior to NT-ProBNP: preserved LVEF, right-sided HF, chronic kidney disease and/or TR. He was a 77-year-old man with a history of COPD, atrial fibrillation, and chronic kidney disease who was admitted with progressive dyspnea and peripheral edemas along with JVD. The patient also presented ascites with elevated intra-abdominal pressure (18mmHg) and bilateral pleural effusion. Blood analysis revealed higher CA125 levels (439U/mL) than previous ambulatory determination (350U/mL), but NT-ProBNP was similar (5233pg/mL at admission; 4938pg/mL in prior visit). Echocardiography showed a dilated RV with systolic dysfunction and severe TR. An intensive diuretic regimen with 250mg of furosemide (continuous intravenous perfusion), hypertonic saline, and 25mg/day of chlorthalidone was initiated. After successful decongestion, CA125 dropped to 75U/mL (at 3-month follow-up). However, NT-ProBNP remained elevated, failing to reflect clinical improvement. The same occurs in patients 4 and 5. Note how NT-ProBNP remained similar to baseline (failing to predict HF-decompensation), while CA125 peaked (Fig. 1). In summary, both biomarkers are complementary and should be used in combination with clinical signs/symptoms and emergent ultrasound techniques to better characterize congestion and help us tailor diuretic therapy in our daily clinical practice.
FundingThis research received no external funding.
Authors’ contributionL. Fuertes Kenneally and S. Villar are equally first authors. All authors have participated in the work and have reviewed and agree with the contents of the article.
Conflicts of interestThe authors declare no conflicts of interest.