Since the publication of the PARADIGM-HF study, sacubitril/valsartan (S/V) has become essential in the treatment of heart failure patients with reduced ejection fraction (HFrEF). In PARADIGM-HF, HFrEF patients treated with S/V experienced a reduction in the risk of sudden cardiac death and all-cause mortality. In fact S/V therapy appears to reduce episodes of ventricular arrhythmia and appropriate implantable cardioverter-defibrillator (ICD) shocks compared with enalapril.1
To date, information regarding the antiarrhythmic effect of S/V is scarce and originates from observational studies of limited sample size.2,3 Therefore, we do not have data on high sudden cardiac death risk populations such as hereditary cardiomyopathies. The aim of this work was to assess the antiarrhythmic effect of S/V in patients with inherited cardiomyopathies with HFrEF and an increased arrhythmic risk.
We prospectively included 28 patients diagnosed with a hereditary cardiomyopathy (after familial and genetic evaluation), at high arrhythmic risk according to the underlying genotype or by the presence of clinical risk markers (previous arrhythmic events, extensive late gadolinium-enhancement or left ventricular systolic dysfunction). The pathogenicity of the variants was established according to physician criteria and the American College of Medical Genetics (ACMG) recommendations.4 We collected clinical, analytical, and echocardiographic data from medical records. Arrhythmia-related variables included premature ventricular contractions density (PVCD), sustained ventricular tachycardia (SVT), non-sustained ventricular tachycardia (NSVT), and ventricular fibrillation (VF) and were obtained from 24-h Holter and/or ICD monitoring. We thoroughly evaluated one year before and after the S/V initiation. Qualitative variables are expressed as absolute frequencies and percentages, and quantitative variables as median and interquartile range [P25-P75]. McNemar's test was applied to qualitative variables and Wilcoxon's test to numerical ones. P<.05 was considered statistically significant. The study protocol was approved by the local ethics committee and all patients included in the study signed an informed consent form.
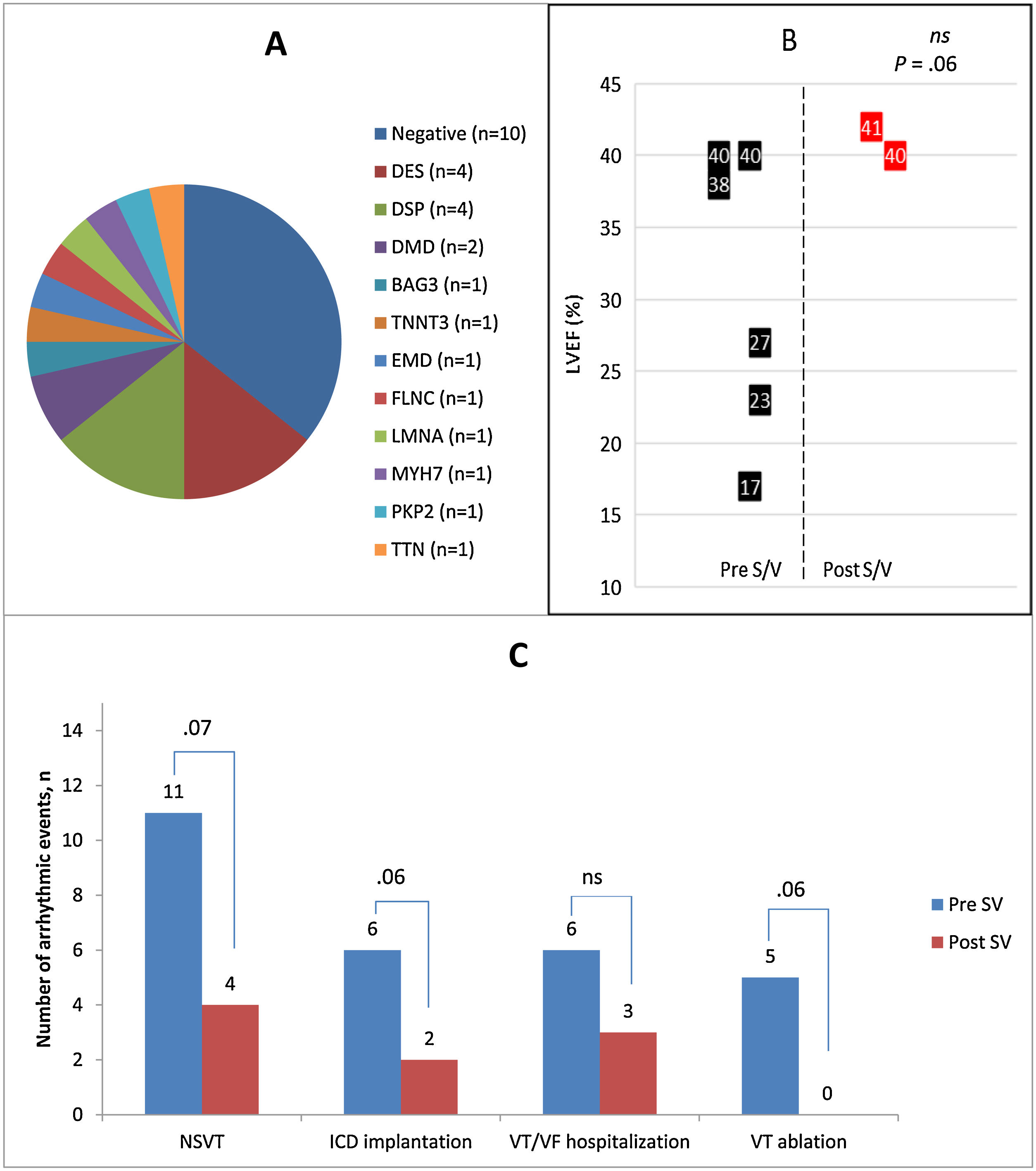
Dilated (42.9%) and arrhythmogenic cardiomyopathy (35.7%) were the most common etiologies (Table 1). Eighteen patients (64.3%) carried a pathogenic genetic variant, being DSP (14.3%) and DES (14.3%) mutations the most common (Fig. 1A). All patients were taking beta-blockers and angiotensin-converting enzyme inhibitors, and 71.4% were under mineralocorticoid receptor antagonist. Six patients were on antiarrhythmic drugs. Antiarrhythmic therapy was not modified in any patient during the study. Seventeen (60.7%) cases were using the optimal S/V dose, presenting 6 patients symptomatic hypotension leading to dose adjustment without discontinuation of S/V.
Baseline characteristics and findings.
| Cardiomyopathy phenotype | |||
| NIDCM, n (%) | 16 (57.1) | ||
| HCM, n (%) | 1 (3.6)) | ||
| ACM, n (%) | 10 (35.7) | ||
| NCCM, n (%) | 1 (3.6) | ||
| Variables | Post-S/V (n=28) | Pre-S/V (n=28) | P |
| NYHA functional class | |||
| I | 0 | 7 (25.0) | .001 |
| II | 22 (78.6) | 21 (75.0) | |
| III | 6 (21.4) | 0 | |
| IV | 0 | 0 | |
| Creatinine (mg/dL) | 0.87±0.22 | 0.89±0.27 | .554 |
| BNP (pg/mL) | 382.13±399.44 | 376.7±360.23 | .910 |
| LVEF (%) | 32.67±7.52 | 37.11±8.61 | .005 |
| PVC, n (%) | 19 (67.8) | 10 (35.7) | .004 |
| PVCD, (%) | 1.65 [0–9.53] | 0 [0–3] | .002 |
| NSVT, n (%) | 11 (39.3) | 5 (17.9) | .07 |
| SVT/VF, n (%) | 7 (25) | 2 (7.1) | .06 |
| Antiarrhythmic therapy | |||
| Beta-blocker, n (%) | 28 (100) | 28 (100) | N.A. |
| Optimal dose, n (%) | 16 (57.1) | 16 (57.1) | N.A. |
| 50%-Optimal dose, n (%) | 4 (14.3) | 4 (14.3) | |
| <50%, n (%) | 8 (28.6) | 8 (28.6) | |
| Amiodarone, n (%) | 1 (3.6) | 1 (3.6) | N.A. |
| Sotalol, n (%) | 5 (17.9) | 5 (17.9) | N.A. |
ACE, angiotensin converting enzyme inhibitors; ACM, arrhythmogenic cardiomyopathy; BNP, brain natriuretic peptide; EV, ventricular extrasystole; HCM, hypertrophic cardiomyopathy; IAD, implantable autonomic defibrillator; LVEF, left ventricular ejection fraction; N.A., not applicable; NCCM, uncompacted cardiomyopathy; NSTV, non-sustained ventricular tachycardia; NIDCM, non-ischemic dilated cardiomyopathy; PVC, premature ventricular complexes; PVCD, premature ventricular complex density; S/V, sacubitril/valsartan; SVT, sustained ventricular tachycardia; VF, ventricular fibrillation.
Data are expressed as no. (%) or mean ± standard deviation or median [interquartile range].
(A) Distribution of identified genotypes; (B,C) Comparative representation of the incidence of severe arrhythmic events (SVT/FV) before and after treatment with sacubitril/valsartan. The black and red squares represent the systolic function of patients with pre-treatment event (n=7) and event during the year following the treatment initiation (n=2). BAG3, Bcl-2-associated athanogene 3; DES, desmin; DMD, dystrophin; DSP, desmoplakin; EMD, emerin; FLNC, filamin C; ICD, implantable cardiac defibrillator; LMNA, lamin A/C; MYH7, myosin heavy chain 7; ns, non-significative; NSVT, non-sustained ventricular tachycardia; PKP2, plakophilin-2; S/V, sacubitril/valsartan; TTN, titin; TTNT3, troponin T3; VT, ventricular tachycardia; VF, ventricular fibrillation.
S/V utilization was associated with a significant improvement in left ventricular ejection fraction (from 32.67±7.5 to 37.11±8.6; P<.01). Regarding the antiarrhythmic effect, we detected a statistically significant reduction in the PVCD in Holter monitoring. There was also a trend towards a reduction in the presence of NSVT, SVT, or VF (Fig. 1B), VT ablations, HF hospitalizations, and ICD implantations (Fig. 1C). Among 14 patients carrying an ICD (57.14% for secondary prevention), 7 patients experienced appropriate shocks during the previous year to S/V initiation. During 1-year of follow-up, only 2 patients presented ICD therapies, independently of left ventricular ejection fraction (Fig. 1B). Hospital admissions were reduced from 6 (4 for VF/SVT and 2 for worsening heart failure to 2 (1 SVT and 1 worsening heart failure), with no statistically significant differences.
Multiple particularities make this cohort of special interest: (a) exclusive inclusion of patients with HFrEF of familial etiology, whose prognosis and arrhythmogenicity is variable depending on the underlying genotype, including very high-risk mutations; (b) average age of patients considerably lower than that of previous studies; and c) long and symmetrical period of analysis (1 year before and after the S/V initiation), which allows a better assessment of the antiarrhythmic effect. Beyond the improvement in cardiac remodeling and functional status, we observed a statistically significant reduction in the PVCD in 24h Holter monitoring. After stratifying by patients who were taking antiarrhythmic drugs, the reduction of PVCD was independent of the presence of these drugs. Previous studies have analysed the reduction of the incidence of PVCD as the amount of PVC per hour, according to ICD algorithm.2,3 This methodology present obvious limitations.
In our cohort we did not observe a statistically significant reduction in the incidence of NSVT or SVT/VF (Fig. 1B), but there was a clear trend to reduction. Previously, 2 major studies, with more heterogeneous and larger samples of patients, observed a reduction of SVT, probably due to larger sample sizes.2,3 Similarly to these studies, in our cohort we observed that the reduction in ventricular arrhythmias was clinically translated into a decrease in the number of arrhythmic episodes and, subsequently, ventricular tachycardia ablations and ICD implantations.
S/V influence profibrotic signaling, wall tension and cardiac remodelling which may contribute to the improved outcomes and, therefore, probably the arrhythmic events.5 These last 2 have demonstrated the activation of membrane channels due to the stretching of the heart fibers. In turn, fibrotic scar contributes to the formation of macro-reentrant circuits and foci of ectopic automaticity.
Our study presents several limitations, such as the unicentric character and the limited sample size. However, our findings are encouraging for the consideration of S/V as a potential antiarrhythmic pharmacological tool in patients with hereditary cardiomyopathies at high arrhythmic risk. In order to obtain robust conclusions, randomized studies with larger sample size should be conducted.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsAll authors declare that they have contributed in any of the following aspects: conception and design of the study, acquisition of data, analysis and interpretation of the data, drafting of the article or critical revision of the intellectual content, or final approval of the version presented.
Conflicts of interestNo conflicts of interest.