Pacemakers have evolved from old-school demand pacing to providing tailored therapies. Nowadays, sophisticated algorithms adapt to changing pacing thresholds and provide an artificial chronotropic response while reducing ventricular pacing and preventing dangerous external interferences, among other functions. This way, pacing has become more physiological, maximizing battery duration and increasing patient safety and quality of life. However, this rising in complexity has made their electrocardiogram harder to interpret. In many cases, these functions may create unusual pacing patterns that could sometimes be mistaken for a device malfunction, prompting unnecessary use of medical resources and patient anxiety that could be avoided if such patterns were recognized. This review presents and explains those different advanced functions and algorithms, classified by their apparent malfunction, as a reference for the general cardiologist.
Los marcapasos han evolucionado de la antigua estimulación a demanda a un tratamiento individualizado. Hoy en día, mediante complejos algoritmos son capaces de adaptarse a cambios en el umbral de estimulación, proporcionar una respuesta cronotrópica artificial, reducir la estimulación ventricular y evitar el peligro de interferencias externas, entre otras funciones. Gracias a ello, la estimulación es más fisiológica, alargando la duración de la batería y aumentando la seguridad y calidad de vida del paciente. Sin embargo, esta complejidad creciente ha hecho que el electrocardiograma sea más difícil de interpretar. En muchos casos estas funciones crean patrones de estimulación inusuales que se pueden confundir con disfunciones, provocando un uso innecesario de recursos sanitarios y preocupación por parte del paciente que podrían evitarse si se reconocieran tales patrones. Esta revisión presenta y explica esos algoritmos y funciones, agrupados según la disfunción aparente, para la consulta por el cardiólogo general.
Every year, some 500000 patients receive a pacemaker in the European Union.1 During the last decades, pacemakers have evolved to be sophisticated devices, far from demand VVI pacing as they were conceived in the sixties. With the intention of being more physiological and provide a patient-tailored therapy, they have incorporated an array of advanced features. Some of these focus on patient safety, such as ventricular safety pacing to prevent cross-talk inhibition, or on pacing threshold measurement to ensure appropriate capture. Others have an impact on patient quality-of-life, such as sensor-driven rate responsiveness to increasing functional capacity, or ventricular pacing (VP) reduction to reduce adverse ventricular remodeling. To give it another twist, other algorithms like transthoracic impedance measurement use sub-threshold currents that some electrocardiogram (ECG) recorders might depict as false pacing artifacts. All in all, these algorithms have in common an apparently abnormal behavior when registered in an ECG. When a pacemaker is expected to work just as single-chamber demand or dual-chamber sequential pacing, the observation of abnormally short (fast) or long (slow) intervals or an apparent failure to sense or to capture, will raise concerns about its normal operation.
In those scenarios, the patient can be given an appointment for an in-person device check-up, but even then, the allegedly abnormal behavior may not be replicated. Only a thorough knowledge of the rules controlling such algorithms may reassure the clinician on discriminating real malfunctions from normal, though complicated, functioning pacemakers. On the other hand, it is virtually impossible to know all the parameters and settings involved in every algorithm for each pacemaker and manufacturer.
Considering that the clinician never knows which particular algorithm is operating when facing an ECG, and that only sees what appears to be a malfunction, a structured approach is paramount. This review is structured according to the malfunction purportedly observed. Then, the different options to such behaviors are explained, including a comprehensive list of the logic ruling the different algorithms from each manufacturer, European and American. Throughout the text, manufacturers are referred to by their current or best known registered trademark: in this regard, it should be noted that ELA changed to Sorin, then to Livanova and lately became MicroPort, Guidant became Boston Scientific, Pacesetter became St. Jude and then Abbott, and Vitatron became Medtronic. With these tools, the clinician will be able to ascertain whether the pacemaker works according to the logic of the algorithm, strange as it may be, or whether it violates any of them, which will indicate a device malfunction.
2Short atrioventricular intervalDual-chamber pacemakers are expected to pace the ventricles some 110–220ms after an atrium is sensed or paced. When an abnormally short atrioventricular interval (AVI) is observed, the relationship between the sensed/paced atrium and the paced ventricle, as well as the AVI value itself, will provide the clue to the underlying function.
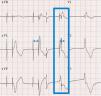
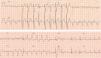
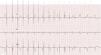
2.1Ventricular safety pacingAtrial pacing pulses might be sensed in the V channel, a phenomenon also known as “crosstalk”. This inappropriate ventricular sensing (VS) will result in ventricular inhibition: should the patient be pacemaker dependent, this ventricular inhibition will cause asystole. Thus, VS in a narrow window following atrial pacing (AP) will trigger a safety pacing to warrant VP and prevent pauses or asystole. However, a ventricular premature beat might also happen just simultaneous with an AP. In this case, if ventricular safety pacing was delivered after a normal AVI, i.e. 200ms, it might pace during a vulnerable period and trigger a ventricular arrhythmia. Therefore, ventricular safety pacing occurs after a short, preset interval (110ms in Biotronik2 and Medtronic,3 120ms or programmed AVI if shorter in Abbott,4 95ms in Sorin5) so that the ventricles are paced and captured, in case of crosstalk, or it falls in a refractory period, in case of a ventricular premature beat (Fig. 1).
Ventricular safety pacing. Dual-chamber pacemaker (in a patient with atrial fibrillation and undersensing of waves). The first two beats show sequential atrioventricular pacing at the programmed atrioventricular interval (AVI) (blue double-headed arrow), with the second being a fusion (narrower than purely paced QRS). The third beat shows atrial pacing simultaneous with a conducted beat sensed in the ventricular channel, thus triggering a ventricular safety pacing with sequential atrioventricular pacing at a much shorter AVI.
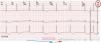
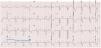
This feature is intended to increase patient safety by guaranteeing ventricular capture6 as well as to reduce battery drain by adjusting pulse amplitude to the lowest value required to maintain capture.7 Some manufacturers (Abbott ACap and RVCap Confirm, Biotronik, Boston Scientific) provide beat-to-beat capture confirmation by assessing the evoked response of the action potential after myocardial capture.8 If capture does not occur in a particular beat, a backup safety pulse of greater amplitude and/or duration is provided. Other devices only perform regular searches of pacing thresholds (Abbott AutoCapture,4 Medtronic, Sorin). When evaluating the ventricular threshold, intrinsic conduction and fusion must be ruled out, so V will be paced after a short AVI. The test VP is followed by a backup VP with higher output to warrant capture in case the testing stimulus fell below the capture threshold (Fig. 2). In this case, regularity is key: threshold measurements are performed by repeating runs of baseline cycles alternating with test cycles (Table 1).
Autothreshold measurement.
| Manufacturer | Mode of operation | Measurement interval | Default setting |
|---|---|---|---|
| Abbott4,9 | Capture is assessed in any case by ER. Bipolar lead required• Atrium (ACap): atrium is paced faster (not above 120bpm) with decreasing output amplitude until 3 LoC beats occur• Ventricle:∘ Beat-to-beat confirmation (RVCap Confirm): backup VP at 63ms when no ER is detected∘ Threshold search (AutoCapture): in DDD, sensed AVI is 25ms and paced AVI is 50ms. In VVI, VP is provided at decreasing amplitudes every beat until two consecutive LoC (with their backup pulses) are detected. However, ventricular rate will not be overridden for the test; hence, if there is spontaneous rhythm, no threshold search will be performed10 | Every 8h | Off |
| Biotronik2,11 | • Atrium: Capture is assessed by sinus reset, pacing in DDI at 20% faster than current rate (up to 108bpm), in decreasing amplitude steps. When 2 AS are detected in a 5-beat window, atrial LoC is declared. The measurement is confirmed by repeating the test, starting at the lowest confirmed amplitude and decreasing in smaller steps• Ventricle (Ventricular Capture Control): Capture is assessed by ER∘ Beat-to-beat confirmation: backup VP at 130ms when no ER is detected∘ Threshold search: In DDD, sensed AVI is 15ms and paced AVI is 50ms. In VVI, there will be pacing 20% faster than the current rate (up to 110bpm). VP is provided at decreasing amplitudes every beat. In the case of LoC, a backup pulse is given. The test is repeated starting at the lowest confirmed amplitude and decreasing in smaller steps to confirm the measurement | Every 24h (at 00:30) | On |
| Boston Scientific12 | PaceSafe. Capture is assessed in any case by ER. Bipolar lead required• Atrium: AP with test pulses at up to 20bpm faster than average atrial rate (but no faster than 110bpm, maximum tracking or sensor rate, whichever is lower). There are no backup atrial pulses. AVI 55–85ms. No beat-to-beat assessment• Ventricle:∘ Beat-to-beat confirmation: backup Vp at 70ms when no ER is detected∘ Threshold search: in DDD, sensed AVI is 30ms and paced AVI is 60ms. In VVI, pacing will occur 10bpm faster than current rate (up to 110bpm or maximum programmed rate, whichever is lower). Pacing amplitude is decreased every 3 beats. After every test pacing pulse over 3.5 V, a backup pulse at 70 ms is provided | Every 21h | On |
| Medtronic13–15 | • Atrium (Atrial Capture Management)∘ If in sinus rhythm but rate <87bpm, capture is assessed by atrial reset. 3 AS are followed by an earlier test AP, and this is followed by two “stabilization” AS. If the test AP does not capture, an atrium will be sensed shortly after that AP∘ If atria are paced but AV conduction is preserved, VS after a test AP will be considered a surrogate of atrial capture. 3 AP cycles at programmed output are followed by a test AP, and this is followed by a support AP (at 1ms pulse width) after 70ms• Ventricle: Capture is assessed by ER. 3 VS/VP support cycles at programmed settings, followed by a test VP, and this is followed by a backup VP after 90ms (series Amplia, Compia), 100ms (series Ensura) or 110ms (series Adapta, Versa, Sensia)• CRT: Each lead is tested pacing separately∘ RV lead is tested as reported above∘ LV lead is tested similarly (3 support VP followed by one test VP) but without backup VP; RV sensing is used to assess LV capture | Every 24h | On |
| Sorin | • Atrium:∘ If sinus rhythm stable and <80bpm, capture is assessed by sinus reset. Every fourth beat is advanced 200ms and paced at increasing amplitudes until no AS happens after the test AP, and the measurement is confirmed by a repeat 4-beat test cycle at the successful amplitude∘ If no sinus rhythm, atrial rate unstable or measurement unreliable, atrial capture is assessed using conducted VS as a surrogate• Ventricle: Capture is assessed by ER. In DDD, AVI is 110ms. In VVI, heart rate will be overdriven up to 95bpm. VP is provided at decreasing amplitudes every beat. In the case of LoC, a backup pulse at 64ms is delivered | Atrium: dailyVentricle: every 6h | Off |
AP, paced atrial beat; AS, sensed atrial beat; AVI, atrioventricular interval; bpm, beats per minute; CRT, cardiac resynchronization therapy; ER, evoked response; LoC, loss of capture; LV, left ventricular; RV, right ventricular; VS, sensed ventricular beat; VP, paced ventricular beat.
Manufacturers’ names are listed in alphabetical order. Figures refer to nominal (standard) settings. Data for Sorin obtained from technical support and on-screen device interrogator help.
This feature is used mainly by Abbott and Biotronik in cardiac resynchronization therapy since it targets to maximize VP. In Abbott devices, the AVI after a VS is shortened for 31 cycles, and if another VS occurs meanwhile, that shortening will be kept for 255 cycles up to 120ms shorter than programmed AVI.4 Biotronik shortens the programmed AVI up to 50ms.2
2.4Dynamic atrioventricular intervalPacemakers can adapt AVI in a physiological way, shortening it at faster heart rates. Usually, both rest (longer) and exercise (shorter) AVI values can be preset and changes between them are made in a linear fashion by the pacemaker; other devices, such as Biotronik, allow for customization of the profile of atrioventricular (AV) adaptation. Thus, an AVI as short as 65ms at high atrial rates can be expected.12
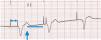
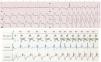
2.5Non-competitive atrial pacingMedtronic pacemakers try to avoid AP shortly after an atrial premature contraction where it might trigger atrial arrhythmia.13 Hence, an AS (atrial sensing) shortly after a ventricle (during the post-ventricular atrial refractory period) will trigger a 300ms interval that might delay the next AP. To balance the cardiac cycle for this delay in AP, the AV interval might be shortened up to a minimum 30ms. Sorin uses the WARAD (Window of Atrial Rate Acceleration Detection) algorithm, whose functioning is more complicated since it depends on the basal rate, timing of atrial premature contraction and existence of previous atrial premature contractions.16 To summarize, WARAD will pace the atria 500ms after an atrial premature contraction, and the ventricle 80ms thereafter (Fig. 3).
3Long atrioventricular intervalA long AVI is usually due to algorithms facilitating intrinsic conduction. They run in a periodic basis, performing rounds to check AV conduction during which abnormal AV intervals can be found. In other cases, they set an apparently abnormal AVI as an operating value.
3.1Atrioventricular hysteresisThis is the simplest form of promoting intrinsic conduction. AV hysteresis algorithms simply lengthen AVI on a periodic basis to allow for existent but slow AV conduction to occur. If there is VS within the extended interval, that new AVI will be kept: otherwise, pacing returns to the preset AVI until the next checking round. In some cases, if no intrinsic conduction is observed after several verifications, the algorithm is disabled until the device is interrogated. These algorithms have shown to reduce VP, while other clinical benefits remain unproven.17 Their main feature, that differentiates them from other V pacing reduction algorithms, is that in AV hysteresis every atrium is followed by a ventricle, either paced or sensed. A comprehensive list of their different registered names and rules of operation is provided in Table 2.
Atrioventricular hysteresis algorithms.
| Manufacturer | Trade name | Rules | Evaluation frequency (search interval) | Default setting |
|---|---|---|---|---|
| Abbott | VIP | • Extends AVI in 100ms for 1 cycle. If there is VS, the extended AVI is kept in effect• Operation suspended when intrinsic A or sensor rate is 110 or faster | Every minute | Off |
| Biotronik | IRSplus | • Extends AVI to 400ms for 10 cycles. If there is VS, the extended AVI is kept in effect | • After a single VS• Every 180 VP | Off |
| Boston Scientific | AV Search+ | • Extends AVI to 300ms for 8 cycles. If there is VS, the extended AVI is kept in effect• Reverts to programmed AVI when ≥2/10V are paced | Every 32 cycles | Off |
| Medtronic | SearchAV+ | • Extends AVI in 62ms for 16 cycles. If there are ≥8/16 VP, AVI is extended other 62ms up to a maximum 170ms more than programmed AVI• If ≥8/16 VS shorter than extended AVI minus 55ms, AVI is shortened in 8ms steps• Permanently suspended after 10 consecutive failed 16-h search attempts | At intervals progressive longer if no intrinsic conduction is found: 15min, 30min, 1, 2, 4, 8 and 16h intervals | Off |
| Sorin | DPlus | • Extends AVI to 350ms• Reverts to programmed AVI after a single VP | • After a single VS.• Every 100 VP, AVI lengthens for 5 cycles• Change from AP to AS, AVI lengthens for 5 cycles | Off |
AVI, atrioventricular interval; IRS, intrinsic rhythm support; VIP, ventricular intrinsic preference; VS, sensed ventricular beat; VP, paced ventricular beat.
Manufacturers’ names are listed in alphabetical order. Figures refer to nominal (standard) settings. Data for Sorin obtained from teaching documents.
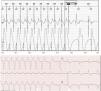
This could probably be the most common pseudo-malfunction encountered in Holter-ECG, since intrinsic AV conduction assessment occurs several times during a 24-h recording. In some cases, they may cause short pauses or atrial cycles without their corresponding ventricle (Fig. 4, Fig. 5, Fig. 6). These algorithms have proved useful to reduce the percentage of VP in patients with relatively preserved AV conduction (i.e. sinus node dysfunction, Wenckebach AV block during exercise or paroxysmal AV block).17 Their different implementations have been proven more effective than AV hysteresis in reducing VP.19,20 They follow different rules depending on the manufacturer (Table 3). As a whole, they operate as atrial-managed modes with VP backup: hence, they are sometimes referred to as AAI↔DDD. When some conditions are met, such as a number of A without V, maximal pause duration or AVI, they switch to a DDD pacing mode. Boston Scientific's Rhythmiq is a particular case thereof.12 It is conceived as AAI(R) operating at a lower rate or sensor rate, plus a backup VVI running behind at atrial rate minus 15 beats per minute (bpm), but neither slower than 30 nor faster than 60bpm (Fig. 5).
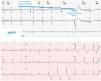
Medtronic Managed Ventricular Pacing. Upper: Holter recording showing sinus rhythm or atrial pacing (asterisk) at a lower rate limit. When an A–A elapses with no conducted QRS, a ventricular pace is delivered at a lower rate limit plus 80ms. Modified with permission from Higueras et al.18 Lower: Pacemaker operating in AAI mode with long (∼600ms) intrinsic AV interval. New Medtronic pacemakers have corrected such behavior.
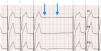
Boston Rythmiq. Patient with a dual-chamber pacemaker programmed at 60bpm. See atrial pacing at lower rate limit with intrinsic atrioventricular conduction at 60bpm (blue line). There is an atrial premature complex (arrow), but it is not followed either by sequential ventricular pacing (VP) after an atrioventricular delay or by VP at lower rate limit. Contrarily, VP occurs at 45bpm (1400ms, dashed red line), that is 15bpm less than lower rate limit. Rythmiq functions as an AAI-managed mode with VVI running in the background at 15bpm slower than lower rate limit.
Ventricular pacing reduction algorithms.
| Manufacturer | Trade name | Switch to DDD | Switch to atrial-based | Default setting |
|---|---|---|---|---|
| Abbott | No such algorithm; only AV hysteresis | |||
| Biotronik | VP suppression | • No VS for 2s (beware that, as for algorithm counters, only VS within 450ms of the last AS/P will be counted)• 2 consecutive atrial blocked events (2 AP/S without VS)• ≥3/8 atrial cycles without Vs• >15 switches to DDD per hour within the last 24h | • Extends AVI to 450ms after a single VS, or after search intervals from 30s to 20h (doubling after each failed check)• Intrinsic AVI ≤450ms for ≥6/8 consecutive VS | • Off |
| Boston Scientific12 | Rythmiq | • ≥3/11 cycles which R–R >150ms longer than P–P (“loss of AV synchrony”)• Vp might occur during atrial-based mode (see text for details) | • As per AV Search + (it must be programmed “On”)• If intrinsic AV conduction for ≥25 cycles and <2/10 last are VP | • Off |
| Medtronic13 | MVP | • ≥2/4 atrial cycles without VS. There is a backup Vp every other A without V, at 80ms after the programmed atrial escape interval• In newer models (Azure and Astra series), long PR is also a criterion for reversion, but this feature is deactivated by default3 | • After 1min (up to 16h, doubling after each failed check) a VP is dropped | • On (active 30min after implantation detection) |
| Sorin16 | SafeRa | • No VS for 3s• 2 consecutive atrial blocked events (2 AP/S without VS)• 3/12 atrial cycles without VS• 6 consecutive AVI longer than 350ms (this value might change at high atrial rates)• If several switches to DDD separated <100 AA cycles, “AV block” is declared | • After 12 consecutive VS• After every 100 VP, except if AV conduction is impaired, in which case it will not be checked until next 8:00am. AV conduction is considered impaired if there are >45 “AV block” during 24h or >15/24h×3 days or >50% DDD for 1h | • On (active 20min after implantation detection) |
AP/S, atrial paced or sensed beat; AV, atrioventricular; AVI, atrioventricular interval; MVP, managed ventricular pacing; VP, paced ventricular beat; VS, sensed ventricular beat.
Manufacturers’ names are listed in alphabetical order. Figures refer to nominal (standard) settings. Data for Biotronik obtained from teaching documents and advertising material.
Many physicians think of a pacemaker as a device that ensures a minimum ventricular rate, usually 60bpm. That is why, when faced to a device pacing at faster rates, even over 100bpm, they are concerned about its normal functioning. However, there are several situations in which it is desirable for the pacemaker to pace faster than that lower rate limit.
4.1Sensor-driven ratePacemakers can become more physiological by increasing their lower rate according to metabolic demands, which is particularly useful in patients with chronotropic incompetence or pacemaker dependency. For that purpose, all current devices use activity sensors based on motion (accelerometers) to measure patient activity and adapt their pacing rate. Besides, some current device families from Boston Scientific (RightRate: Accolade, Essentio, Proponent and Altrua 2) and Sorin (TwinTrace: Reply, Kora and Symphony), as well as legacy Medtronic Kappa, use blended sensors that integrate information from a minute ventilation sensor.21 This minute ventilation uses sub-threshold pacing pulses to measure cyclic changes in transthoracic impedance to estimate the respiratory rate and adapt their rate accordingly. In any case, activity sensors can increase the pacing rate up to a preset value, usually in the 120–130bpm range. The operation of the widely extended accelerometer sensor can be verified by “fooling” the sensor by gently tapping on the device for a few seconds.
4.2Rate smoothing or ventricular rate regulationIn some circumstances, a sudden drop in heart rate might occur, as in paroxysmal AV block or a pause following atrial tachycardia in bradycardia–tachycardia syndrome. Other rhythm disorders such as atrial fibrillation might be symptomatic from the very irregularity of heart rhythm. Rate smoothing adapts the pacing rate not to a lower fixed rate but to a percentage of the previous R–R interval, thereby softening any abrupt changes in heart rate (Fig. 7).
Rate smoothing. The upper tracing shows atrial fibrillation conducted with rapid ventricular rate. A rate smoothing algorithm does not allow the heart rate to drop abruptly, so lower rate limit is temporarily increased to allow minimum deviations from previous heart rate. If such algorithm is turned off, as in the lower tracing, sudden changes in heart rate may occur when pacing only at lower limit. Modified with permission from Ruiz Pizarro et al.22
To measure ventricular thresholds, there must be VP. Therefore, some manufacturers (Boston Scientific, Biotronik) overdrive intrinsic heart rate, up to a maximal predefined value (usually maximum tracking or sensor rate or 110bpm) (Fig. 8 and Table 1). A backup pace is usually provided to prevent asystole in case of loss of capture during these measurements (Fig. 2).
Threshold search in a Boston pacemaker under atrial fibrillation. See rapid ventricular response (narrow, irregular QRS) overdriven by the pacemaker (wider, regular QRS) for some seconds until intrinsic heart rhythm is resumed. These tests occurred every 21h: see the time stamp on the left, over 3 consecutive days.
In vasovagal and carotid sinus syncope, a component of hypotension is added to bradycardia. Thus, a pacemaker merely working at a lower rate might not overcome the hemodynamic depression and would not avoid syncope. Different algorithms (Table 4) have been devised to detect the sudden bradycardia that precedes syncope and pace at a faster rate (intervention rate) aiming to compensate hypotension with tachycardia and to alleviate symptoms (Fig. 9). With the same spirit but with a different rationale, Biotronik's CLS (Closed Loop Stimulation) does not depend on a previous drop in heart rate. It uses sub-threshold current to measure myocardial inotropy during the cardiac cycle and estimate sympathetic tone, according to which pacing rate is adapted, modifying cardiac output and closing the loop of autonomic feedback.23
Sudden bradycardia response.
| Manufacturer | Trade name | Requisites | Intervention |
|---|---|---|---|
| Abbott | No such algorithm; only an adaptation using rate hysteresis and a higher lower rate interval | ||
| Biotronik | No such algorithm; only Closed Loop Stimulation | ||
| Boston Scientific12 | SBR | • Available in DDD pacing mode• As for >1min followed by >10bpm drop and atrial pacing for 3 beats• Inhibited during rest (as per minute ventilation sensor) | Pacing at sensor rate or previous atrial rate plus 20bpm, for 2min |
| Medtronic3 | RDR | • Available in DDD, AAI↔DDD and DDI modes• Heart rate falls by ≥25bpm within less than a minute to a minimum rate of 60bpm | Pacing at 100bpm for 2min, then rate is reduced over 5min until intrinsic rhythm appears or lower rate limit is reached |
| Sorin | Rate acceleration | Not really a dedicated algorithm for sudden bradycardia. Likewise to Abbott's, rate hysteresis is programmed to delay pacing, kicking in at a transient higher-than-normal lower rate interval | |
AS, sensed atrial beat; bpm, beats per minute; RDR, rate drop response; SBR, sudden brady response.
Manufacturers’ names are listed in alphabetical order. Figures refer to nominal (standard) settings.
After raising a great hype in the 90s, these algorithms fell from widespread use. Their goal is to prevent atrial tachyarrhythmia by keeping a constant pace in the atria: however, their usefulness is yet to be proven.24 For instance, atrial preference pacing overdrives intrinsic sinus rhythm. After any spontaneous AS, Boston's pacemakers will accelerate heart rhythm by shortening the cardiac cycle by 10ms, and intrinsic heart rate will be searched by lengthening the cardiac cycle 10ms every 4 cycles.12 Abbott, Biotronik (accelerates 8bpm, searches every 20 cycles),2 Medtronic (30ms shortening, searches decreasing 20ms every 20 cycles),13 and Sorin have similar functions. Other algorithms overdrive atrial rhythm after an episode of atrial tachyarrhythmia or prevent post-extrasystolic pause by shortening the cycles following an atrial premature contraction.
4.6Fallback response or automatic mode switchingIf atrial tachycardia or fibrillation is detected, the pacemaker switches to a non-tracking mode, i.e. VDI/DDI (R). In some cases, to compensate for the reduction in cardiac output secondary to the loss of active atrial emptying, the lower ventricular rate will be increased to 70bpm (Boston12), 80bpm (Abbott4) or 10bpm over the lower rate limit (Biotronik2). This mode switching relies on appropriate detection of atrial tachycardia (also known as atrial high rate episodes, AHRE), since it is triggered by a predefined number of fast sensed atria (over 1602–18012 AS per minute). Thus, if AS is inaccurate, AHRE will not be recognized and irregular tracking and pacing of the atria may occur. This is to be suspected whenever the ECG shows atrial and VP at irregular intervals.
4.7Antitachycardia pacingThis behavior belongs to implantable, transvenous, cardiac defibrillators. Ventricular tachycardia can be terminated either by high-energy shocks or by transiently pacing faster than the tachycardia, taking control over it (“entraining”) and terminating it. Ventricular spikes can be seen regularly spaced, i.e. pacing at a constant speed (burst), or in decreasing distance, i.e. in accelerating progression (ramp) (Fig. 10). The programming of anti-tachycardia pacing (type, rate, duration, etc.) is outside of the scope of this review. Anyway, it can be suspected when pacing is of short duration and unusually fast (over 150bpm and even up to 270bpm).
Antitachycardia pacing in an implantable cardioverter defibrillator. The tracing starts with ventricular tachycardia that prompts an anti-tachycardia pacing (ramp, see how the spikes are progressively faster up to a minimum 200ms), followed by transient acceleration and extinction of the arrhythmia, resuming normal ventricular pacing.
To avoid the deleterious effect of VP, the device allows an intrinsic heart rate lower than the minimum pacing rate. In other words, it will wait for the heart rate to fall to a lower value before pacing, but that pacing will start at a faster rate than the intrinsic one.
5.2Ventricular pacing reduction algorithmsThey are described in section above.
5.3Sleep/rest modeThe lower rate is decreased during some prespecified hours (Biotronik, Medtronic) or under some levels of patient activity (Abbott, Sorin), further mimicking the physiological chronotropic response. This function is programmed off by default. If activated, note that rate is adapted to the internal device clock: should the patient travel to a different time zone, the pacemaker will go to sleep at an inconvenient local time.
5.4Endless-loop tachycardia treatmentIn endless-loop tachycardia treatment (ELT), a tachycardia is established in the presence of VP, retrograde ventriculoatrial conduction and AS that triggers another VP, perpetuating a reentrant tachycardia where the AV node is the retrograde limb and the pacemaker acts as the antegrade limb. ELT must be suspected in dual-chamber pacemakers when there is VP at upper tracking rate (120–130bpm) with no AP (Figs. 11 and 12).
Endless loop tachycardia (ELT), onset. The electrocardiogram strip (above) starts with sinus rhythm and ventricular pacing, followed by a premature atrial contraction (arrow) that is tracked to the ventricles with a longer AVI (199ms) not to violate the upper rate interval (130bpm, 460ms). This ventricular pace propagates retrogradely to the atrium and starts an ELT at the upper rate interval. Device interrogation (below) shows in detail such phenomenon (from upper to lower: marker channel, internal clock timing, atrial electrogram, and ventricular electrogram). This patient was shown to have 1:1 ventriculoatrial conduction at rates over 160bpm.
Endless loop tachycardia (ELT), termination. Device interrogation (above) shows atrial sensing (AS) tracked to the ventricles (VP) during tachycardia until one of the atria is discarded (AR): the next pacing shows sequential atrioventricular pacing (see text for details). The electrocardiogram (below) shows the characteristic sudden drop in heart rate.
To declare an ELT, as opposed to appropriate tracking of sinus tachycardia, the device might cross-check the appropriateness of that tachycardia with the information provided by the activity sensor.13 Other devices3,4 use an elegant approach: the timing of a VP is changed to confirm the presence of ventriculoatrial linking and ELT. In either case, the interruption of the antegrade limb will terminate the episode. An AS is not tracked, so a VP is withdrawn,2,5,12,13 the loop is broken and the heart rhythm drops back to normal. Abbott devices pace sequentially atrium and ventricle, with the AP occurring at 330ms after the last AS4 (Fig. 12).
6Failure to sense6.1Magnet modeA thorough review of pacemaker behavior under magnet application has been published elsewhere.25 As a summary, a pacemaker responds to magnet application by pacing in asynchronous mode (V00) at a preset rate that depends on the manufacturer and battery status. In other cases, when previously programmed so, magnet application will elicit no apparent response, either because “Magnet mode” has been turned off or because the device has been configured to store an internal electrogram. There are a couple of notable exceptions to this rule: as preset, Biotronik devices will function in asynchronous mode only for 10 beats and then return to their normal parameters; and some Abbott and Pacesetter legacy devices (i.e. Microny) have a function named VARIO that performs a threshold test with 16 VP at 100bpm and constant output followed by 15 VP at 120bpm with progressive reduction in pacing voltage (Fig. 13).
6.2Magnetic resonance imaging or electrocautery modeMany current pacemakers have a dedicated magnetic resonance imaging, or electrocautery mode. It is essentially an asynchronous mode to prevent interference and oversensing that may lead to inappropriate inhibition or tracking. Upon activation, it can usually be programmed to any manually selected rate. Some devices will revert to previous pacing mode after a preset time, and others will even detect the magnetic field and enter “magnetic resonance imaging mode” only when approaching the gantry.
6.3Ventricular safety pacingA premature ventricular beat coincidental with an AP could elicit a ventricular safety pacing with a pacing spike right on the QRS, giving an impression of undersensing. Nothing farther from the truth: that spike is a safety mechanism already described above.
6.4Noise reversionDifferent pacemakers recognize patterns of repetitive signals at high frequencies (over 20Hz for Sorin, 25Hz for Boston, 100Hz for Abbott). When such signals are sustained long enough, the device turns to an asynchronous mode at lower rate or sensor rate. The cause and management of such interference, from electromagnetic interference to myopotential oversensing, is beyond the scope of this review.
6.5Triggered ventricular pacing (VVT mode)Cardiac resynchronization therapy devices always try to maximize VP. So, when there is a ventricular premature beat or supraventricular rhythm conducted to the ventricles, there will be VS. Some cardiac resynchronization therapies will pace from the left ventricular lead in response to this VS in the right ventricular channel, willing to resynchronize as much as possible (Fig. 14). It can be differentiated from true failure to sense because the pacing spike appears earlier than expected at the programmed pacing rate.
Triggered ventricular pacing. The patient had a cardiac resynchronization therapy functioning in VVIR mode at ∼70bpm with high density ventricular premature beats (asterisks). The cardiac resynchronization therapy responded to sensing of every ventricular premature beat by pacing from the left ventricular lead: this triggered pacing occurs, therefore, earlier than expected (arrow). Reproduced with permission from Fernández-Vega et al.26
Digital ECG recorders have band-pass filters to remove baseline respiration and movement wander, myopotentials and electrical powerline noise. However, these filters also suppress pacemaker signals, so current ECGs do not represent “true” pacing spikes but they rather depict an artificial pacing spike when they find a signal with a particular bandwidth, voltage, and duration criteria.27 Similarly, any artifact meeting such criteria will be signaled as a pacing stimulus, which can be a nuisance to the clinician. Some pacemakers have features that use sub-threshold pulses to obtain information about the device or the patient; such pulses might be recorded in the ECG, showing a regular though un-understandable behavior with the appearance of failure to sense/capture (Fig. 15). In other cases, just random, ambient noise can be picked by the ECG recorder. There is no common feature or pattern that can be recognized in these cases, but we must be aware of such possibility.
False pacing spikes. The electrocardiogram (ECG) of a patient with a single-chamber Biotronik Lumax ICD showed pacing spikes after every QRS (upper ECG); a recording was later repeated, activating manually ventricular pacing, with a clearly different QRS morphology (middle ECG). In this case, the false pacing spikes were due to the home monitoring intrathoracic measurement that sends 1024 sub-threshold pulses every hour, 100ms after every ventricular event (sensed or paced). The lower ECG shows a trace of a patient with a dual-chamber Sorin Kora: AAI-based pacing can be observed, with spikes randomly distributed (asterisks) throughout the tracing. Simultaneous device interrogation showed no pacing other than atrial.
As explained before, some pacemakers perform a regular assessment of their capture threshold (Table 1). By definition, this means that some pacing artifacts will not capture, but usually, a backup pace will then be provided. However, Medtronic cardiac resynchronization therapy devices are somewhat different. They assess the capture threshold daily using iterations of three support pulses followed by one test pulse. Right ventricular test pulses are followed 90ms later by a backup pulse to ensure capture, as opposed to left ventricular test pulses, which lack such backup.14 Therefore, loss of capture during the assessment of left ventricular threshold will cause a pause in the absence of intrinsic atrioventricular conduction or escape rhythm.
8ConclusionsThis review tries to cover the different pacemaker algorithms that may mimic a pacemaker failure when its operation is observed in a conventional ECG, Holter or telemetry monitoring. We believe that it may help the clinician to understand the apparently atypical behavior associated with these algorithms, and distinguish it from true device dysfunctions. However, bear in mind that some of the algorithm parameters (timings, intervals) can be manually modified, and new devices and firmware are released every year, impeding their recognition. Nevertheless, the bottom line is that pseudodysfunctions always follow the same patterns, as opposed to real malfunctions, which are genuinely chaotic.
9Conflicts of interestNone relevant to this article.
Abbreviations: AS, atrial sensing; AP, atrial pacing; AVI, atrioventricular interval; ELT, endless loop tachycardia (pacemaker-mediated); VS, ventricular sensing; VP, ventricular pacing.