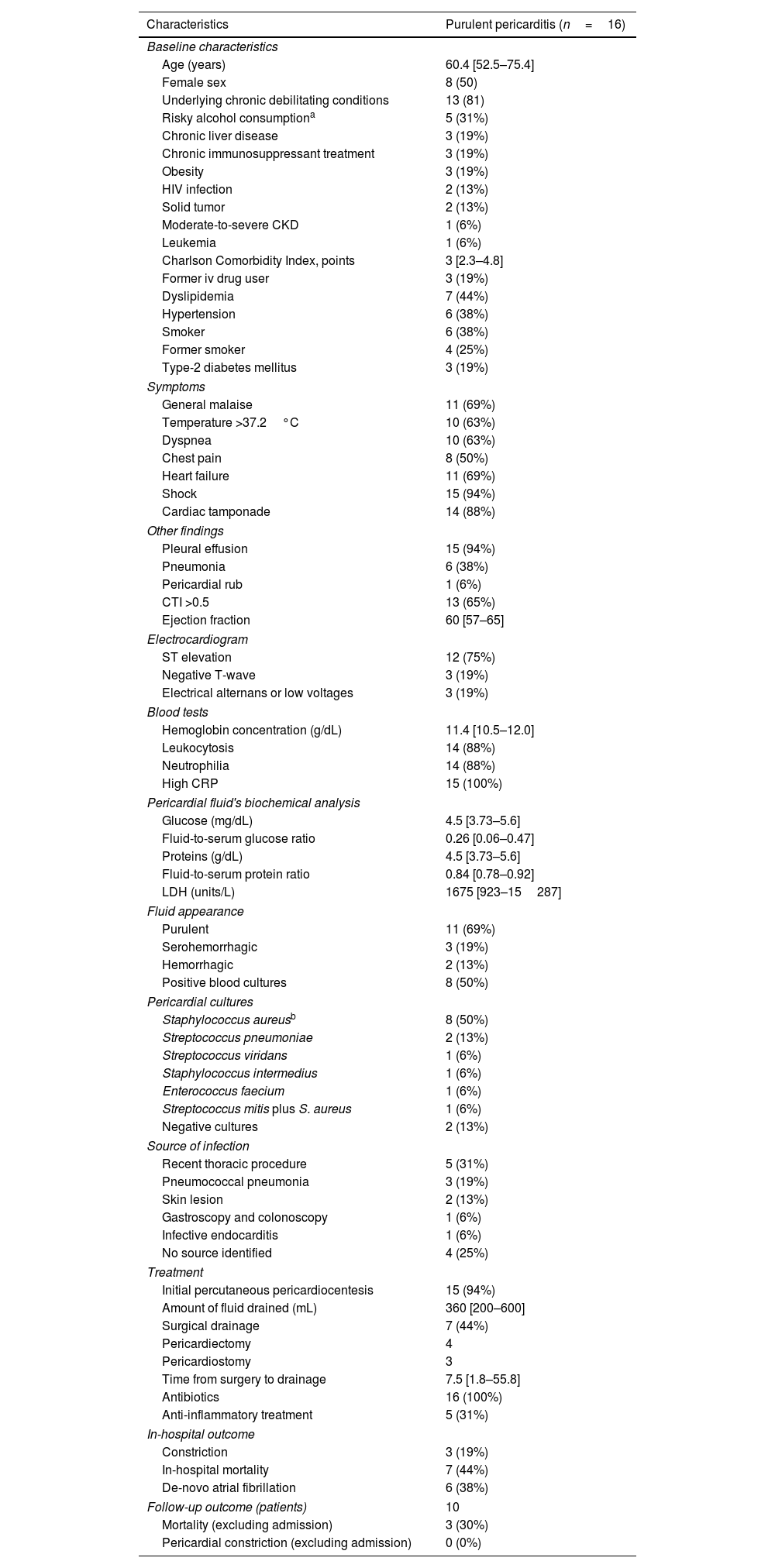
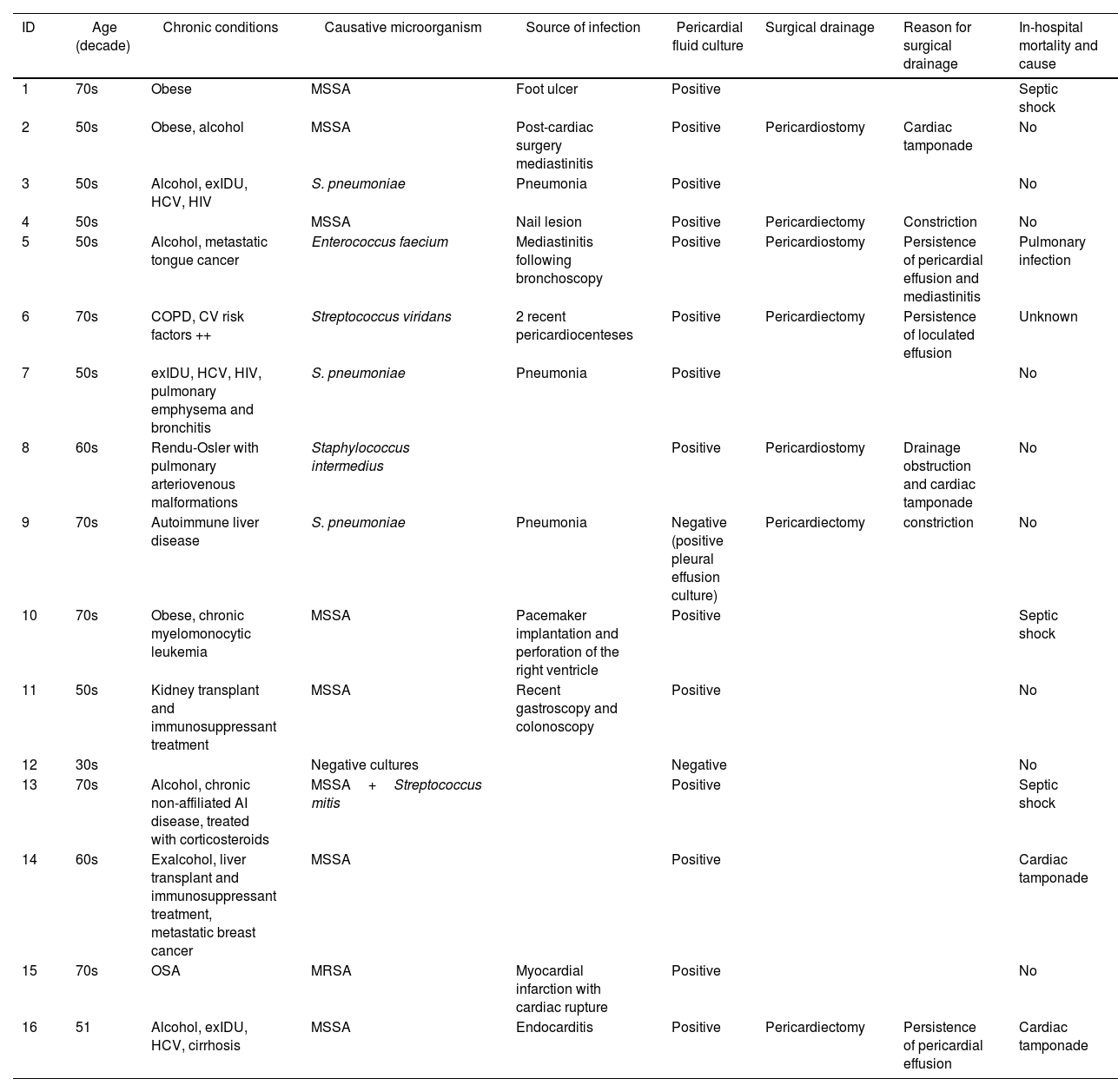
Artículo
REC: CardioClinics
Use datos de acceso a SEC en el menú Acceder.
Si es socio de la Sociedad Española de Cardiología y no puede acceder con sus claves, escriba a rec@cardioclinics.org.
Use the Society's website login and password here.
If you are member of SEC and you have some problems with your login data, please contact with rec@cardioclinics.org.
Si ya tiene sus datos de acceso, clique aquí.
Si olvidó su clave de acceso puede recuperarla seleccionando la opción "He olvidado mi contraseña".Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora